PTWI expressed as Sn including stannous chloride. Iron Fe and water Iron and water.
Answered Iron Iii Chloride Is Reduced By Bartleby
Drinking water may not contain more than 200 ppb of iron.

Iron chloride and tin reaction. Exploding a tin can using methane. Seawater contains approximately 1-3 ppb of iron. To be sure reaction was complete small excess of the SnCl 2 is added and then solution is treated with mercury II chloride.
Iron nails Copper foil 2 inch by 18 inch or copper wire Zinc foil 2 inch by 18 inch Aluminum foil 2 inch by 18 inch Tin foil 2 inch by 18 inch Magnesium ribbon 2 inch Magnesium ribbon 1 inch piece 01 phenolphthalein 01 g to 50-50 water-alcohol mixture 01 M potassium ferricyanide K. Iron metal is more electropositive than copper and therefore will displace it from its salts. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website.
When metallic iron oxidation state 0 is placed in a solution of hydrochloric acid ironII chloride is formed with release of hydrogen gas by the reaction Fe 0 2 H Fe 2 H 2. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. It is corrosive to metals and tissue.
Ferrous chloride is a greenish white. The third class contains metals such as chromium iron tin and lead which react only with strong acids. Create a small explosion in this demonstration by electrolysing water to produce hydrogen.
Most algae contain. Iron chloride FeCl2 More. Reaction mechanisms environmental impact and health effects.
The primary aim of the cards is to promote the safe use of chemicals in the workplace. Tin is a post-transition metal in group 14 of the periodic table. The 1988 PTWI was not reconsidered and was maintained at the fifty-fifth meeting 2000.
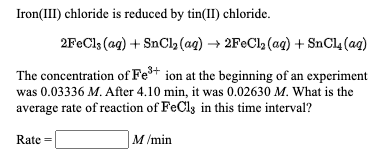
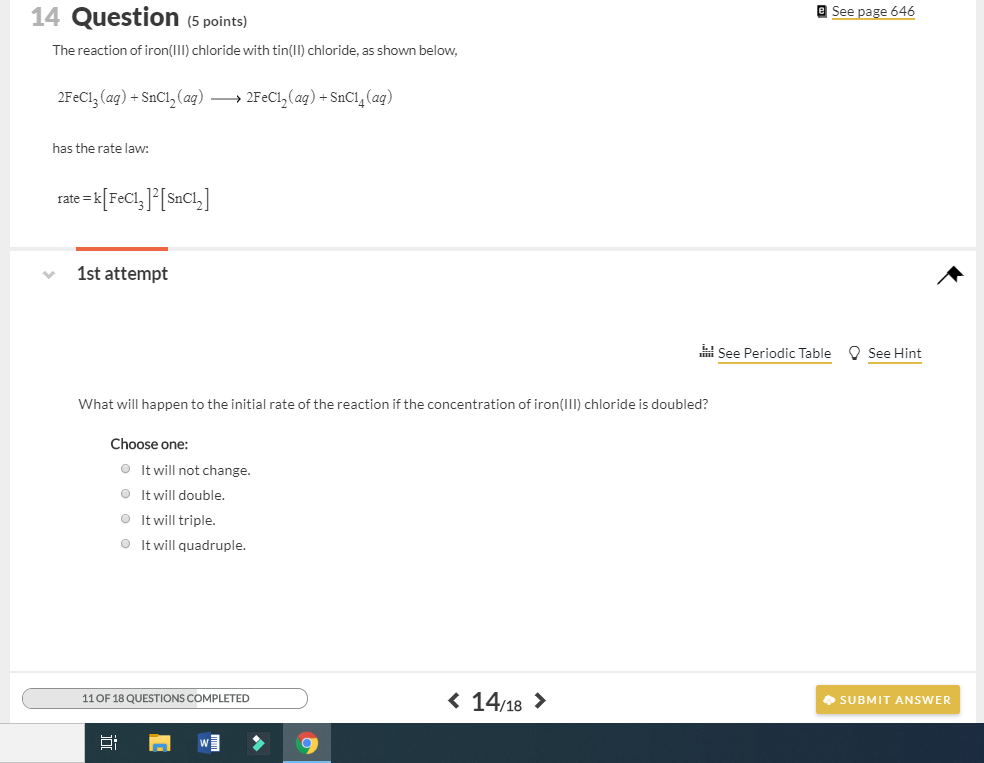
Click to see our best Video content. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic structure. 2FeCl 3 SnCl 2 2FeCl 2 SnCl 4.
The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and. TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in.
In association with Nuffield Foundation. When iron metal is exposed to air and water usually it turns into rust a. The acute toxicity of tin was assessed at the fifty-fifth meeting but data were insufficient for establishing an acute reference dose.
Use this demonstration to illustrate how methane can create an explosive mixture with the oxygen in air. Simple S N 2 reaction. Tin-plated tiffin boxes are utilised because they.
Tin is non-toxic and its reactivity is lower than that of iron. Properties of the element. In association with Nuffield Foundation.
Rusting of iron can be prevented by tinning. S N 1 and S N 2. Ferrous chloride solution is the greenish-white crystalline solid dissolved in water.
It is obtained chiefly from the mineral cassiterite which contains tin dioxide. Tin plating is usually used for making tin cans as tin is very expensive. The first alloy used on a large scale was.
The Committee reiterated the conclusion reached in 1988 that tin concentrations as low as 150 mgkg in canned beverages. Tin is nontoxic ductile malleable and adapted to all kinds of cold-working such as rolling spinning and extrusionThe colour of pure tin is retained during exposure because a thin invisible protective film of stannic oxide is formed spontaneously by reaction with the oxygen of the air. The chemical symbol for Tin is Sn.
Rivers contain approximately 05-1 ppm of iron and groundwater contains 100 ppm. Food cans are tinned which implies that they have a thin layer of tin on them. Allyl Chloride with HS S N 2 Reaction.
This is our newest publication and has been created to support the school technician profession in Scotland. Take A Sneak Peak At The Movies Coming Out This Week 812 Lin-Manuel Miranda is a Broadway and Hollywood Powerhouse. Metals in the fourth class are so unreactive they are essentially inert at room temperature.
Why does the reaction of iron and copper chloride occur Why does the reaction of iron and copper chloride occur. These metals are ideal for making jewelry or coins because they do. The ironII hydroxide is then further oxidised by oxygen to form hydrated iron.
Why does the reaction of iron and copper chloride occur. It also contains even less active metals such as copper which only dissolves when treated with acids that can oxidize the metal. As a result when an electroplated thin coating of tin metal is deposited on iron and steel items the iron and steel objects are protected from rusting.
2 o Benzyl Chloride with HS S N 2 Mechanism. S N 2 Reaction. It is used in dyeing in medicine and sewage treatment.
The low melting point of tin and its firm adhesion to clean surfaces of. Includes kit list and safety instructions. This is done by dipping the iron into molten tin or by electroplating an iron sheet using tinIV chloride as the electrolyte.
The amount varies strongly and is different in the Atlantic and the Pacific Ocean. It is possible to reduce iron 3 using Jones reductor column filled with granules of amalgamated zinc however most commonly reduction is done with tin II chloride. Stability and structure of carbocations.
S N 2 examples. Substrate structure controls substitution mechanism S N 1 or S N 2. Exploding bubbles of hydrogen and oxygen.
Thus the overall redox reaction is as follows. Fe 0 Cu 2 Fe 2 Cu 0. Benzyl Chloride with HS S N 2 Reaction.
After tin plating the inside of the can is coated with a thin.

A Level Chemistry 9701 22 F M 16 Q2 Cher Questions Solution Bank

Solved The Reaction Of Iron Iii Chloride With Tin Ii Chegg Com

Solved Iron Iii Chloride Is Reduced By Tin Ii Chloride The Chegg Com

Tin Reacts With Strong Acid But With Weaker Acid It Does Not
Solved The Reaction Of Iron Iii Chloride With Tin Ii Chegg Com
Ferric Oxidation Lanthanumk S Blog
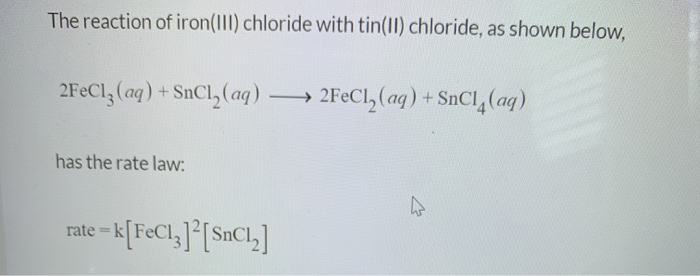
Solved The Reaction Of Iron Iii Chloride With Tin Ii Chegg Com

Chemical Reactions Reactions Can Actually Be Categorized In The Following 3 Main Groupings 1 The Precipitation 2 The Redox 3 The Acid Base Synthesis Ppt Download
