How can calcium hydroxide be prepared. Therefore this compound is a base.

Is Cao Acidic Basic Or Neutral Youtube
CaCO 3 HEAT CaO CO 2.

Is calcium oxide a base. Carbon 1200 C 4397. Cement production alone. One calcium compound lime calcium oxide CaO was extensively used by the ancients.
Ionic bonds are atomic bonds created by the attraction of two differently charged ionsThe bond is typically between a metal and a non-metal. The pure calcium carbonate occurs in two crystalline forms. Lubricants and lubricant additives.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. A cement base of ZOE or glass ionomer cement is placed gently over the MTA and allowed to set. It is heated to form quicklime CaO which is then added to water H 2 O.
Talc synthetic fluorphlogopite nylon-12 triisostearin phenyl trimethicone magnesium stearate dimethicone silica caprylyl glycol calcium sodium borosilicate synthetic wax cetyl dimethicone calcium aluminum borosilicate hydrogenated polydecene trimethylsiloxysilicate perlite alumina hditrimethylol hexyllactone crosspolymer tin oxide magnesium silicate - may. The carbonation finally results in thermodynamically stable calcium carbonate products. High-calcium quicklime provides superior chemical performance.
The main target users are workers and those responsible for occupational safety and health. The pH scale is used to measure acidity and alkalinity. Al2O3 H2SO4 Al2SO43 H2O.
Litmus is a common indicator. Calcium carbonate CaCO 3 is one of the common compounds of calcium. Quicklime is produced by heating limestone as shown in the following equation.
G3311 2 mayb expert wear es reno high pearl ingredients talc triisostearin hydrogenated polydecene magnesium stearate synthetic wax calcium sodium borosilicate synthetic fluorphlogopite hditrimethylol hexyllactone crosspolymer caprylyl glycol silica calcium aluminum borosilicate alumina perlite tin oxide magnesium silicate - may contain peut contenir mica ci 77891 titanium dioxide ci. Calcite is the most common. The calcium oxide CaOH 2 has many applications in which the hydroxyl ion is necessary.
Aluminum hydroxide decomposes to produce aluminum oxide and water. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. Calcite hexagonal shaped which possesses birrefringent properties and aragonite rhombohedric.
A 2 AlOH 3 Al 2O3 3 H2O b Decomposition 11. This forms another material known as slaked lime CaOH 2 which is an inexpensive base material used throughout the chemical industry. Molecular Weight 10009 gm Calcium 4004 Ca 5603 CaO.
It is the fifth most abundant element in Earths crust and the third most abundant metal after iron and. The Raw Calcium formula provides all the necessary co-factors such as 50mcg of Vitamin K2 in the form of MK-7 1600IU of Raw Food-Created vitamin D3 and. General Calcite Information.
1 minute The curing of Concrete elements by diffusing carbon-di-oxide into it under controlled pressure and temperature is one of the popular methods of accelerated curing. Calcium oxide that survives processing without reacting in building products such as cement is called free lime. The name for the element was taken from the Latin word for lime calx.
A small proportion of it dissolves when combined with water forming a solution known as limewater. Acids can react with some metals to form a salt. Quicklime is created by heating limestone as shown in the following equation.
Reaction with hydrochloric acid. The silvery rather soft lightweight metal itself was first isolated 1808 by Sir Humphry Davy after distilling mercury from an amalgam formed by electrolyzing a mixture of lime and mercuric oxide. It is a white alkaline and crystalline solid.
Calcium oxide CaO commonly known as lime or quicklime is a widely used chemical compound. Some so-called calcium phosphates contain oxide and. Periodic trends of the oxides.
The structure of the bond is rigid strong and often crystalline and solid. Chalk marble and limestone are all forms of calcium carbonate. A 3 CaCO 3 2 AlPO 4 Ca 3PO 42 Al 2CO 33 b Double Replacement 10.
This is a neutralisation reaction. Corrosion inhibitors and anti-scaling agents. Calcium oxide CaO used in the pulp manufacturing.
Graveling and road bed material. Due to electrolyte dissociation this compound liberates OH- ions. Vitamin Code Raw Calcium is the first raw calcium formula composed of AlgaeCal RAW a certified organic plant source of calcium derived from the marine algae Algas calcareas rich in naturally occurring minerals and trace minerals.
Both it and a chemical derivative calcium hydroxide of which quicklime is the base anhydride are important commodity chemicals. The ICSC project is a common undertaking between the World Health Organization WHO and. Zinc reacts with silver nitrate to.
Changing from a low- to a high-calcium diet at 14 weeks of age or later however caused no detrimental effects on performance. MgO a good thermal conductor and electrical insulator that is used in firebrick and thermal insulation and calcium oxide CaO also called quicklime or lime used extensively in the steel industry and in water purification. The process lets the CO2 to diffuse into the concrete and undergo carbonation.
Aluminum oxide reacts with hot dilute hydrochloric acid to give aluminum chloride solution. The natural carbonates are. A question arises about the best time to switch pullets from a low-calcium growing diet to a high-calcium laying diet.
The acetylene is the base material of a great number of important chemicals for the organic industrial chemistry. Calcium is a chemical element with the symbol Ca and atomic number 20. Aluminum oxide contains oxide ions and thus reacts with acids in the same way sodium or magnesium oxides do.
Chemical is part of scrap metaliron kish used in the manufacture of metal in an electric arc furnace. The primary aim of the cards is to promote the safe use of chemicals in the workplace. Ionic bonds also melt at high temperatures.
Calcium hydroxide is formed by the action of water on calcium oxide also called slaked lime CaOH2. Calcium phosphate is a family of materials and minerals containing calcium ions Ca2 together with inorganic phosphate anions. Engineered fill for construction.
Alkaline solutions turn red litmus blue and acid solutions turn blue litmus red. As an alkaline earth metal calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. In this case it acts as a base.
Feeding a diet with 325 percent calcium starting at 50 days of age increased the incidence of urolithiasis in later life Wideman et al 1985. Quicklime is relatively inexpensive. Common examples include sodium chloride potassium iodide calcium carbonate and copper sulfate.
Calcium carbonate reacts with aluminum phosphate to produce calcium phosphate and aluminum carbonate. This same variation is observed in the reaction of oxides with water and the resulting acid-base character of the products. Indicators are substances that change colour with a change in acidityalkalinity.
Cohens Pathways of the Pulp Tenth Edition 2011.
This way we can calculate the molar mass of a compound or one-carbon compound. If the molar mass of the salt is 218 gmol what mass is required.

A Sample Of Calcium Bromide Contains 0 2 G Calcium And 0 8 G Bromine By Mass Calculate The Empirical Brainly Com
Molar Mass of Frequently Calculated Chemicals.
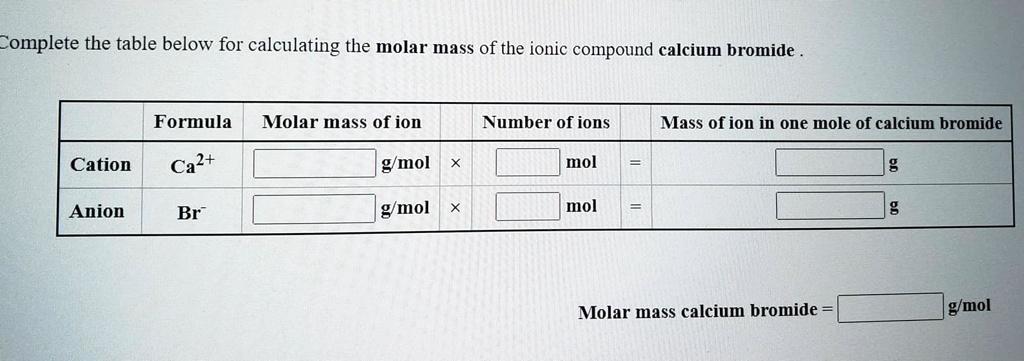
Molar mass of calcium bromide. Well add those numbers together along with the unit grams per mole for finding molar mass. Based on the chemical formula of a substance we know the composition of the substance. Since sodium carbonate contains one carbon atom two sodium atoms and three oxygen atoms the molecular weight is.
Atomic mass of Carbon 1201. What is the formula mass amu of calcium phosphate. The dissolution stoichiometry shows a 11 relation between moles of calcium ion in solution and moles of compound dissolved and so the molar solubility of CaOH 2 is 69 10 3 M.
The identity of a substance is defined not only by the types of atoms or ions it contains but by the quantity of each type of atom or ion. Check Your Learning The K sp of PbI 2 is 14 10 8. How do you know the Order of Elements in a Chemical Formula What is Chemical Formula.
Use the value ksp14x10-8 for PbI2 to solve the following problems. What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml. Molar Mass Is 9896G.
Atomic mass of Oxygen 1600. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula.
230 x 2 46. Carbon2427 12 202. Molecular mass or molar mass are used in stoichiometry calculations in chemistry.
Molar absorptivity of all-trans retinol in ethanol at 325 nm is ɛ 52770 M 1 cm 1. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Molar absorptivity of all-trans retinoic acid at 350 nm in ethanol ɛ 45300 M 1 cm 1.
For example water H 2 O and hydrogen peroxide H 2 O 2 are. These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. What is the molar mass of sodium carbonate Na2CO3.
Now we have to divide all the values with the lowest obtained value. It is defined to be 112 of the mass of one atom of carbon-12 and in older works is also abbreviated as amu. Individual compounds include the anhydrous material x 0 the hexahydrate x 6 and the rare dihydrate x 2.
How many grams are in 379 moles of calcium bromide CaBr 2. Molar mass of Carbon Monoxide 2801. Calculate the molar mass of.
How many grams of H_3PO_4. If you need a 15 M solution of calcium bromide eqCaBr_2 eq and have 850 grams of solid eqCaBr_2 eq how many milliliters of solution can you make. Calcium bromide is the name for compounds with the chemical formula Ca Br 2 H 2 O x.
To Calculate Empirical Formulae first we have to divide the given percentages of atoms by their molecular masses. Path length L 1 cm for standard cuvette. That leads to this.
Click here to see a video of the. Hydrogen 407 1 407. All are white powders that dissolve in water and from these solutions crystallizes the hexahydrate.
The standard molar entropy associated with calcium oxide corresponds to 40 joules per mole kelvin. As defined in the ICE table x is the molarity of calcium ion in the saturated solution. In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses.
What is the mass solubility of calcium sulfate in pure water expressed in gL. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Ksp24x10-5 for calcium sulfate.
This compound is known to emit an intense glow when it is heated to temperatures above 2400 degrees celsius. How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution. 120 x 1 12.
Hydrogen 407 201 2. If molecular formula calculator add up the total value which is 12 46 48 106. CaOH 2 SO 4 CaSO 4 H 2 O.
Will precipitation occur when you add 005 mL of 010 M KBr to a saturated solution of AgCl. Also important in this field is Avogadros number N. MM millimolar millimoles per litre 10-3 moles per litre µM micromolar micromoles per litre 10-6 moles per litre nM nanomolar nannomoles per litre 10-9 moles per litre pM picomolar picomoles per litre 10-12 moles per litre fM femtomolar femtomoles per litre 10-15 moles per litre.
What is the molar solubility of calcium sulfate in pure water. A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present in the substance. Calcium phosphate Ca 3 PO 4 2 is an ionic compound and a common anti-caking agent added to food products.
Chlorine7165 355 201. λ max of all-trans-retinoic acid in ethanol is 350 nm. We can also use molecular weight calculator for finding molar mass of a.
Soluble in water glycerol. A solution of calcium bromide contains 200 g dm-3. Therefore the molar mass of Na2CO3 is 106 gmol.
16 x 3 48. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. A the anesthetic halothane C 2 HBrClF 3 b the herbicide paraquat C 12 H 14 N 2 Cl 2 c caffeine C 8 H 10 N 4 O 2 d urea CONH 2 2 e a typical soap C 17 H 35 CO 2 Na.
The hydrated form is mainly used in some drilling fluids. What is the molar. How many grams are in 0572 moles of glucose C 6 H 12 O 6.
Calculate the molar mass of each of the following. MV mass molar mass x 100 L 200 g 199886 gmol x 0100 M When CaBr 2 ionizes two bromide ions are released for every one CaBr2 that dissolves. In the NaOH.
Now use the number of moles and multiply it by the molar mass. Molarity moles per litre of solution M Commonly used terms include. Carbon202 2.
What volume of 12 M HCl solution is needed to prepare 5 liters of 00250 M solution. What is the molarity of the solution with respect to calcium bromide and bromine ions.
Zinc gluconate is a zinc salt of gluconic acid comprised of two gluconic acid molecules for each zinc cation 2. It is an odorless light blue or blue-green crystal or powder which is easily soluble in water and insoluble in ethanol.
File Calcium Gluconate 2d Skeletal Png Wikimedia Commons
Selenium as Selenium Selenate 70 mcg 127.
The formula of calcium gluconate. Studies to establish the level of calcium provided by human milk are long-standing in nature and little information has emerged to change the conclusions of earlier analyses. It contains no antimicrobial agent. Low levels can lead to osteoporosis and calcium deficiency disease or hypocalcemia.
A millimole is the amount of a substance equal to its atomic weight expressed in milligrams. If more than 5 mg fluoridekg body weight ie more than 23 mg fluoridelb body weight have been ingested induce vomiting give orally soluble calcium eg milk 5 calcium gluconate or calcium lactate solution and immediately seek medical assistance. The modified Myers cocktail which consists of magnesium calcium B vitamins and vitamin C has been found to be effective ag.
6 Provide the following information to the EMS team andor physician. Vitamin B-6 as Pyridoxine HCI 2 mg L-Arginine 3 g Korean Asian Ginseng Root Extract aerial part and root 100 mg American Ginseng root. A The concentration of the hydrofluoric acid and its MSDS.
Calcium gluconate decreases levels of ferrous gluconate by inhibition of GI absorption. For obvious reasons care must taken to order the correct formulation of calcium. Inpersol with 425 dextrose.
This patented formula helps support sexual desire and improves sexual performance and staying power. Calcium carbonate can cause a decrease in the absorption of Methylene blue resulting in a reduced serum concentration and potentially a decrease in efficacy. We believe that you should be able to trust anything that you plan to eat and should know exactly what it is that youre putting in your body.
Building on the work of the late John Myers MD the author has used an intravenous vitamin-and-mineral formula for the treatment of a wide range of clinical conditions. A continuous infusion of 50 ml calcium gluconate in 450 ml D5W can also be used starting at 10 mlmin titrating to desired ionized calcium level. In medicina trova impiego come integratore di calcio e regolazione del battito cardiaco così come nelliperpotassiemia come cardioprotettivo una sua alternativa.
Ferrous gluconate increases levels of calcium gluconate by enhancing GI absorption. Phosphorus is measured in millimoles because at a physiologic pH of 74 the phosphate ion exists partly in divalent and partly in monovalent forms. The Folexin formula not only contains biotin fo-ti and folic acid but more than 20 other vitamins minerals and herbs to create one potent hair formula.
The side-benefit of this nutrient rich formula is that it also supports healthy skin nails and lashes. Zinc gluconate is a generally recognized as safe GRAS substance by FDA. The Myers cocktail Altern Med Rev.
Find 7647-01-0 and related products for scientific research at MilliporeSigma. The ratio is 4 mM of hydrogen phosphate divalent HPO 4 2- to 1 mM of dihydrogen phosphate monovalent H 2 PO 4. As Zinc Gluconate 15 mg 136.
Applies only to oral form of both agents. Intravenous calcium comes in two forms calcium gluconate 10 ml vial 94 mg elemental calcium or calcium chloride 10 ml vial 273 mg elemental calcium. 0125 to 025 mgmL.
Therefore vigorous rinsing with water may be recommended after brushing and before rinsing if. Calcium saccharate tetrahydrate 45 mg. 5 Calcium gluconate gel 25 should be re-applied or Zephiran soaking repeated every 10-15 minutes until the ambulance arrives or a physicianEMT gives medical treatment.
GG is a supplement company with a difference. Hydrochloric acid andor sodium hydroxide may have been added for pH adjustment 60 to 82. Calcium is a vital mineral that strengthens your teeth bones and even your heart function.
Calcium as Calcium Carbonate 270 mg 21. However fractional calcium absorption is lower in formula-fed infants averaging about 40 percent among different formula types Abrams et al 2002. Il gluconato di calcio è un composto chimico sale di calcio dellacido gluconico di formula C 6 H 11 O 7 2 Ca.
Although the composition of milk varies significantly from the start. Calcium gluconate 94 mg. EI - Estimative Index EI daily EI Low Light Weekly PPS Pro - Perpetual Preservation System PMDD - Poor Mans Dosing Dupla Drops Dose to reach a target and Result of your dose.
Applies only to oral form of both agents. For example some dentifrice ingredients like calcium hydroxide or aluminum hydroxide can form a complex with fluoride ions and reduce a mouthwashs effectiveness. Viene impiegato come additivo alimentare il cui codice identificativo secondo le norme dellUnione europea è E578.
We support clinicians patients and communities at large. For accidental ingestion of more than 15 mg fluoridekg of body weight ie more than 69 mg fluoridelb body weight induce vomiting. 10 calcium gluconate solution given intravenously is the form of calcium most widely used in the treatment of low blood calciumThis form of calcium is not as well absorbed as calcium lactate and it only contains 093 930 mgdl calcium ion defined by 1 g weight solute in 100 ml of solution to make 1 solution wv.
Each mL of Calcium Gluconate Injection contains 100 mg of calcium gluconate equivalent to 94 mg of calcium gluconate and 45 mg of calcium saccharate tetrahydrate hydrochloric acid andor sodium hydroxide for pH adjustment 60 to 82 and sterile water for injection qs. It is available as a trace mineral supplement and over the counter as a lozenge form for a reduced duration of common colds and with decreased symptom severity. Medline is more than your business partner.
Calcium Gluconate Injection USP is a sterile nonpyrogenic supersaturated solution of calcium gluconate for intravenous use only. Water for injection qs. B Date time of exposure duration.
Do not add solutions of Cefepime for injection to solutions of ampicillin at a concentration greater than 40 mg per mL or to metronidazole vancomycin gentamicin tobramycin netilmicin sulfate or. Medical uses Low blood calcium. We believe that quality is paramount when it comes to vitamins minerals and any supplements.
Calcium as calcium carbonate monobasic calcium phosphate. Welcome to our website and discover more about us. Milligrams using this formula.
Calcium carbonate can cause an increase in the absorption of Methylphenidate resulting in an increased serum concentration and potentially a worsening of adverse effects. Nutrient Dosing Calculator v1591. Manganese as manganese gluconate 05 mg 22 Chromium as chromium picolinate 10 mcg 29 Sodium as sodium bicarbonate monobasic sodium phosphate 65 mg 3 Potassium as potassium bicarbonate potassium carbonate monobasic potassium phosphate 200 mg 4 Percent Daily Values are based on a 2000 calorie.
Although it has been nasally administered for. Cefepime for Injection Admixture Incompatibility. Kidney COP Calcium Oxalate Protector 120 Capsules Patented Kidney Support for Calcium Oxalate Crystals Helps Stops Recurrence of Stones Stronger Than Chanca Piedra Stone Breaker Supplements 44 out of 5 stars 1956 ratings.
Well show you. Copper gluconate is the copper salt of D-gluconic acid. Aminosyn II 425 with electrolytes and calcium.
Planted Aquarium Nutrient Dosing Calculator.
Calcium sulfate dihydrate. Reaction with Oxygen.
How To Balance Ca Br2 Cabr2 Calcium Bromine Gas Youtube
MgBr 2 4H 2 O.

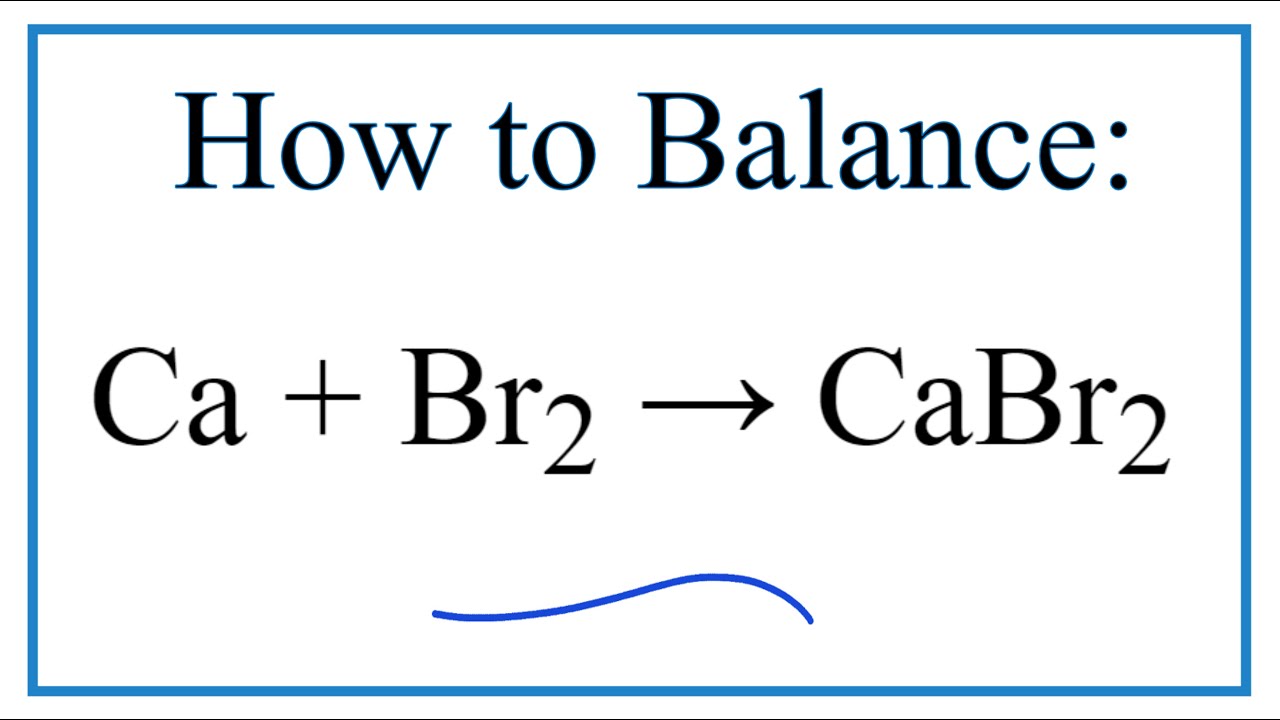
Formula for calcium bromide. Pinaverium bromide is a medication used for functional gastrointestinal disorders. Binary Ionic Formula Practice Name_____ 1 sodium chloride Na1 Cl-1 NaCl 2 lithium bromide Li1 Br-1 LiBr 3 magnesium flouride Mg2 F-1 MgF2 4 potassium oxide K1 O-2 K2O 5 calcium sulfide Ca2 S-2 CaS 6 aluminum iodide Al3 I-1 AlI3 7 barium bromide Ba2 Br-1 BaBr2 8 aluminum sulfide Al3 S-2 Al2S 3 9 calcium phosphide P-3 Ca2 P-3. Sulfur dioxide ____SO2_____ 2.
Ca 2 Calcium. Chemical formulas can be. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
Cd3N2 cadmium nitride 19. Calcium sulfur oxide dihydrate. Ionic Compounds Naming and Formula Writing.
SnS tin II sulfide 8. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3. Solubility Product Constants near 25 C.
Sodium thiosulfate ____Na2S2O3_____ 3. Give the formula for the following. Ammonium phosphate formula NH 4 3 PO 4.
Metals lose electrons to produce positve ions called cations. E-mail to a friend. Chemical formula plays an important role in understanding different concepts of chemistry.
Optical constants of CaCO 3 Calcium carbonate Calcite Ghosh 1999. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Some elements form stable groups or polyatomic ions that combine with other elements to form compounds.
Then identify the anion and write down its symbol and charge. MnBr 2 4H 2 O. PbO2 lead IV oxide 56.
A monatomic meaning one-atom. CaSO4 In this compound the SO4 is called a sulfate group. HgNO 3 2 2H 2 O.
Al 3 Aluminum. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. Ultrapure water Fundamental Research Samples containing protein Titration anionic and.
Write the chemical formula for each of the following compounds. MnBrO 2 2 4H 2 O. Be 2 Beryllium.
In this video well walk through this process for the ionic compound calcium bromide. For example the formula for magnesium bromide. Iodide bromide and cyanide in electroplating baths Titration of anionic and cationic surfactants in washing powders soaps ph also 10 Titration of cationic surfactants Sulfide Low conductivities eg.
Ca3N2 calcium nitride 9. ONE-SCHOOLNET Periodic Table. HgS mercury II sulfide 13.
NaCIO4 sodium perchlorate 47. Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box. Calcium bromide is the name for compounds with the chemical formula Ca Br 2 H 2 O x.
Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. 000607 mol O. Finally combine the two ions to form an electrically neutral compound.
First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula. Calcium VA - trace analysis Back titration of excess Ba with EDTA Special applications Nitrate. Now use the number of moles and multiply it by the molar mass.
Binary Ionic Compounds Type II The cation of a transition metal is always named first like any cation and the anion second. CuO copper II oxide 15. All are white powders that dissolve in water and from these solutions crystallizes the hexahydrate.
Sodium chloride NaCl and magnesium oxide MgO. A 212 g of potassium bromide KBr b 01488 g of phosphoric acid H 3 PO 4 c 23 kg of calcium carbonate CaCO 3 d 78452 g of aluminum sulfate Al 2 SO 4 3 e 01250 mg of caffeine C 8 H 10 N 4 O 2. Moles to Grams Conversion Formula Questions.
The entire group 1 metal can react with oxygen to form metal oxide. Hg2I2 mercury I iodide 10. To find the formula of an ionic compound first identify the cation and write down its symbol and charge.
E-mail to a friend. Its condensed formula is CH 2 CCNCOOCH 3. CaF2 calcium fluoride 14.
You should complete this by Sunday. Complete these in lab and on your own time for practice. They contain sodium bromide up to 1000 mgampoule or calcium bromide up to 800 mgamp.
It is most effective when taken for a full course of treatment and is not designed for immediate symptom. Cu3P2 copper II phosphide 16. An example of this is the sulfate polyatomic ion in the compound calcium sulfate.
The transfer of electrons between metals and non-metals produces charged particles called ions. C 9 H 20 is the chemical formula for nonane. Synthesis structure and.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. MnBr 4 4H 2 O. An ionic compound is composed of a metal and a non-metal.
Ionic Compound Naming and Formula Writing List 1. Calcium sulfide dihydrate. What is the correct molecular formula for the compound manganese bromide tetrahydrate.
It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping to restore the normal contraction process of the bowel. Copy this to my account. A 25-year-old female suffered from forgetfulness and unstable gait after long-term frequent injections of a preparation to relieve head and neck pain.
SnS2 tin IV sulfide 18. Fe2CrO43 iron III chromate 46. Use the stock form for the transition metals.
Ammonium carbonate formula NH 4 2 CO 3. MnBr 2 3H 2 O. ZnBr2 zinc bromide 45.
What is the correct molecular formula for the compound mercuryI nitrate dihydrate. MgBr2 tells us that two bromine atoms combine with one magnesium atom. Glue Cyanoacrylate 5 5 2.
A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another. Zinc iron II iron III gallium silver lead IV chloride ZnCl 2. How many grams are in 379 moles of calcium bromide CaBr 2.
Potassium carbonate K2CO3 22. Na Mg 2 Non. Hg2Cl2 mercury I chloride 57.
SnSO42 tin IV sulfate 55. PbBr2 lead II bromide 12. C 5 H 5 NO 2 is the chemical formula for methyl cyanoacrylate one of the molecules used as the main component of cyanoacrylate glues.
Ammonium nitrate formula NH 4NO 3 90. KCIO potassium hypochlorite 48. 000152 mol H 3 PO 4.
PbO lead II oxide 11. KCl potassium chloride 17. The names are found by finding the intersection between the cations and anions.
Ionic Compound Formula K sp. Copy this to my account. Reaction of Group 1 Elements.
Chemical Formula Nomenclature Practice. Formula Type Chemical Name CaO I Calcium oxide C 2 H 2 M Dicarbon dihydride LiOH I Lithium hydroxide SO 3 M Sulfur trioxide H 3 PO 4 A Phosphoric acid MgCl 2 I Magnesium chloride HNO 3 A Nitric acid Ca 3 P 2 I Calcium phosphide ZnCO 3 I Zinc carbonate NaNO 3 I Sodium nitrate H 2 SO 3 A Sulfurous acid AgCl I Silver chloride CH 4 M Carbon tetrahydride Cu 2 C 2 O 4 I Copper I oxalate SnI 4 I Tin. It consists of one sulfur atom.
Ag Silver. Sodium nitrite NaNO2 31. CsBr cesium bromide 7.
ZnCH3COO2 zinc acetate 58. Al 2 S 3. Individual compounds include the anhydrous material x 0 the hexahydrate x 6 and the rare dihydrate x 2.
The hydrated form is mainly used in some drilling fluids. Blood tests showed hyperchloremia 171 mEqL and a negative anion gap -487 mEqL.
Chemical Formula Nomenclature Practice. You should complete this by Sunday.
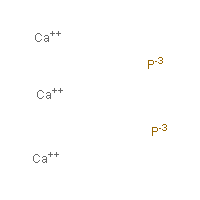
Calcium Phosphide Hazardous Agents Haz Map
FeI3 iron III iodide 32.
Formula of calcium phosphide. Simple Binary Ionic Compounds Please complete the following table. What is the correct name for the compound P 4 S 3. Al2O3 aluminum oxide 37.
Name of Ionic Compound. Type II Metal Non-Metal In general it is NOT possible to use the periodic table to predict what cations ie what the charge on the cation is are formed by transition metals or the main group metals that. Click here to check your answer to Practice Problem 5.
Metals lose electrons to produce positve ions called cations. AgBr silver bromide 29. Review some examples of cations or positive ions.
Complete these in lab and on your own time for practice. Use the stock form for the transition metals. An ionic compound is never formed between two cations only or two anions only.
Sulfur dioxide ____SO2_____ 2. Give the formula for the following. 7 VO2 vanadium IV oxide.
Write the formulas of the following chemical. Hg2O mercury I oxide 35. The positive charge more protons versus electrons for a cation is shown by a number and plus sign after the formula.
Copy this to my account. The key to writing proper ionic formulas is simple. The transfer of electrons between metals and non-metals produces charged particles called ions.
6 IO2 iodine dioxide. 616 Write the symbols for the ions and the correct formula for the a. Write the chemical formula for the following ionic compounds.
2 provides a dispersion formula based on data from ref. 4 CrCO33 chromium VI carbonate. Fe2O3 iron III oxide 28.
Covalent and write the appropriate formula for it. An example of a group 1 phosphide is sodium phosphide Na 3 POther notable examples include aluminium phosphide AlP and calcium phosphide Ca 3 P 2 which are used as pesticides exploiting their tendency to release toxic phosphine upon hydrolysis. Potassium phosphide K 3P zinc carbide Zn 2C manganese IV sulfide MnS 2 cobalt.
Cesium phosphide __Cs3P__ calcium iodide _CaI2_ barium fluoride __BaF2__ magnesium nitride __Mg3N2__ aluminum bromide __AlBr3__ sodium selenide _Na2Se. I Calcium phosphide ZnCO 3 I Zinc carbonate NaNO 3 I Sodium nitrate H 2 SO 3 A Sulfurous acid AgCl I Silver chloride CH 4 M Carbon tetrahydride Cu 2 C 2 O 4 I Copper I oxalate SnI 4 I Tin IV iodide PbS 2 I Lead IV sulfide CO 2 M Carbon dioxide Al 2 SO 4 3 I Aluminum sulfate. 3 C2Br6 dicarbon hexabromide.
Zn 3 P 2 is a II-V semiconductor with a direct band gap of 15 eV and may have applications in photovoltaic cells. Formula Type Chemical Name HCl A Hydrochloric Acid Hg 2 SO 4 I Mercury I sulfate N 2 O 3 M Dinitrogen trioxide CdS I. 10 N2O3 dinitrogen trioxide.
Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. A proper ionic formula has a cation and an anion in it. Data Expressions for n CSV - comma separated TXT - tab separated Full database record Optical transmission calculator.
Formula of Ionic Compound s 2. An ionic compound is composed of a metal and a non-metal. Notice that cations keep their name sodium ion calcium ion while anions get an -ide ending chloride ion oxide ion.
LiBr lithium bromide 39. Ca3P2 calcium phosphide 25. Formula 21 sodium phosphide Na 3 P 22 magnesium nitrate MgNO 3 2 23 lead II sulfite PbSO 3 24 calcium phosphate Ca 3 PO 4 3 25 ammonium sulfate NH 4 2 SO 4 26 silver cyanide AgCN 27 aluminum sulfide Al 2 S 3 28 beryllium chloride BeCl 2 29 copper I arsenide Cu 3.
Calculate the value of x if the formula of hydroxyapatite is Ca x PO 4 3 OH. Zn3P2 zinc phosphide 34. Ionic Compound Naming and Formula Writing List 1.
Write the symbols for the ions and the correct formula for the ionic compound formed by each of the following. A second compound exists in the zinc-phosphorus system zinc diphosphide ZnP 2. Na2N sodium nitride 26.
5 CaCO3 calcium carbonate 6 NiPO4 nickel III phosphate 7 Li2SO3 lithium sulfite 8 Zn3P2 zinc phosphide 9 SrC2H3O22 trontium acetates 10 Cu2O copper I oxide 11 Ag3PO4 silver phosphate 12 YClO3 yttrium chlorate 13 SnS2 tin IV sulfide 14 TiCN4 titanium IV. Binary phosphides include phosphorus and one other element. 11 dinitrogen trioxide N2O3.
Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. Zinc phosphide Zn 3 P 2 is an inorganic chemical compoundIt is a grey solid although commercial samples are often dark or even black. What is the correct name for the compound SeF 6.
Cs3N cesium nitride 33. Metals that can form more than one ion will have their positive charge denoted by a roman numeral in parenthesis immediately next to the name of the Polyatomic. If theres just a plus sign it means the charge is plus 1.
Calcium and chlorine b. Gallium and oxygen. Predict the formula of calcium phosphate which contains Ca 2 and PO 4 3-ions.
E-mail to a friend. Sodium chloride NaCl and magnesium oxide MgO. Ca2 Calcium S2-Sulfide Sr2 Strontium Se2-Selenide Ba2 Barium Zn2 Zinc Cd2 Cadmium 3 Al3 Aluminum 3- N3- Nitride P3- Phosphide Table 2 Metals That Form More Than One Monatomic Ion ELEMENT ION FORMULA SYSTEMATIC NAME COMMON NAME Chromium Cr2 Chromium II Chromous Cr3 Chromium III Chromic.
PbF2 lead II fluoride 31. Binary Ionic Formula Practice Name_____ 1 sodium chloride Na1 Cl-1 NaCl 2 lithium bromide Li1 Br-1 LiBr 3 magnesium flouride Mg2 F-1 MgF2 4 potassium oxide K1 O-2 K2O 5 calcium sulfide Ca2 S-2 CaS 6 aluminum iodide Al3 I-1 AlI3 7 barium bromide Ba2 Br-1 BaBr2 8 aluminum sulfide Al3 S-2 Al2S 3 9 calcium phosphide P-3 Ca2 P-3. Potassium and sulfur b.
SnO tin II oxide 27. Rubidium and bromine. Aluminum Al 3 barium Ba 2 bismuth Bi 3 cadmium Cd 2 calcium Ca 2 cesium Cs chromium III Cr 3 cobalt Co 2.
Sodium thiosulfate ____Na2S2O3_____ 3. Aluminum and iodine d. 5 Ag3P silver phosphide.
Calcium carbonate 26 NiPO 4 nickel III phosphate 27 Li 2 SO 3 lithium sulfite 28 Zn 3 P 2 zinc phosphide 29 SrC 2 H 3 O 2 2 strontium acetate 30 Cu 2 O copper I oxide 31 Ag 3 PO 4 silver phosphate 32 YClO 3 yttrium I chlorate 33 SnS 2 tin IV sulfide 34 TiCN 4 titanium IV cyanide 35 KMnO 4 potassium permanganate 36 Pb 3. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. Magnesium phosphide Mg 3 P 2 also is moisture sensitive.
The total positive charge must balance the total negative. The bone and tooth enamel in your body contain ionic compounds such as calcium phosphate and hydroxyapatite. 8 PbS lead II sulfide.
Chemical formulas for ionic compounds are called ionic formulas The chemical formula for an ionic compound. Li2S lithium sulfide 30. Zinc carbonate ZnCO 3 aluminum hypochlorite Aℓ C ℓO 3 calcium phosphate Ca 3PO 4 2 cadmium phosphate Cd 3PO 4 2 iron III sulfate Fe 2SO 4 3 mercury II chlorite HgC ℓO 2 2 potassium phosphite K 3PO 3 magnesium hydroxide MgOH 2.
SrCl2 strontium chloride 36. Sodium and nitrogen c. FeI2 iron II iodide 38.
Na Mg 2 Non. P3-Phosphide ClO 4-Perchlorate As3-Arsenide BrO-Hypobromite Type II Cations Name BrO 2-Bromite Fe. It is used as a rodenticide.