Name the following compounds. 185 Occurrence Preparation and Compounds of Hydrogen.
Magnesium 2 Hydrogen Phosphite Get Quote
Six rabbits revealed slightly irritating effects.
Hydrogen phosphite formula. Ph3 Ph3 Phosphane Hydrogen Phosphide Phosphorus Hydride Phosphorated Hydrogen Phosphorus Trihydride PH3 Molar Mass PH3 Oxidation Number Disodium Hydrogen Phosphite - Na 2 HPO 3 Na2HPO3 Molar Mass Na2HPO3 Oxidation Number. The prefix hypo removes one more oxygen from the ite ion and the prefix per adds an additional oxygen to the ate ion. 183 Structure and General Properties of the Metalloids.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Triethyl phosphite showed slightly irritating effects on the eyes of 3 male rabbits. C 25 H 42 N 7 O 17 P 3 S xLi CAS No.
It is a conjugate base of a hydrogenphosphite. RS Na CH 3 2 CHBr CH 3 2 CHSR Na Br. Formula and the correct name of the chemical compound.
Together they comprise a single ion with a 1 charge and a formula of ceNH_4. The formula of an ionic compound containing a polyatomic ion must have a charge balance that equals zero 0. The carbonate ion consists of one carbon atom and three oxygen atoms and carries an overall charge of 2-.
Tin IV sulfate SnSO42 24. The compounds can be prepared by methanolysis of phosphorus trichloride or by heating diethylphosphite in methanol. All animals showed slight or clearly visible conjunctival erythema in the treated eyes.
83762 free acid basis Compare Product No. Hydrogen sulfate or bisulfate HSO 4-hydrogen sulfite or bisulfite HSO 3-hydrogen phosphate HPO 4 2-hydroxide OH-hypochlorite ClO-nitrate NO 3-nitrite NO 2-oxalate C 2 O 4 2-perchlorate ClO 4-permanganate MnO 4-peroxide O 2 2-phosphate PO 4 3-phosphite PO 3 3-silicate SiO 3 2-sulfate SO 4 2-sulfite SO 3 2-thiocyanate SCN-thiosulfate S 2 O 3 2-. Cesium hydrogen sulfite CsHSO3 18.
Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N. Silver dichromate Ag2Cr2O7 25. Bicarbonate HCO 3.
The atoms of a polyatomic ion are tightly bonded together and so. Thiolate conjugate bases are easily formed and have proven to be excellent nucleophiles in S N 2 reactions of alkyl halides and tosylates. Iron III hydroxide FeOH3 27.
SbNO 2 3 antimony III nitrite HIO hypoiodous acid NH 4 2CO 3 ammonium carbonate LiC ℓO 4 lithium perchlorate HC ℓO 2 chlorous acid HCH 3COO acetic acid Au 3PO 3 gold I phosphite Cu 3BO 3 copper I borate HNO 2 nitrous acid H 3PO 3 phosphor ous acid. It is a colorless extremely poisonous and flammable liquid that boils slightly above room temperature at 256 C 781 FHCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver Sulfate AgBr Silver Bromide AgCh3COO Silver Acetate AgCl Silver Chloride AgF SilverI Fluoride AgNO3 Silver Nitrate AlNO33 Aluminium Nitrate AlOH3 Aluminium Hydroxide Al2CO33 Aluminum Carbonate Al2CrO43 Aluminum Chromate Al2SO43.
Dimethylphosphite is an organophosphorus compound with the formula CH 3 O 2 POH. Name or write the formula for the following polyatomic ions sulfate CO3 2-. Slight chemosis and increased secretion were recorded in two animals each.
Mercury II phosphite Hg3PO32 19. CH 3 COO C 2 H 3 O 2. Hydrogen carbonate bicarbonate HCO3-hydrogen sulfate bisulfate HSO4-hydrogen phosphate biphosphate HPO4 2-hydroxide OH-hypochlorite ClO-iodate IO3-nitrate NO3-nitrite NO2-oxalate C2O4 2-perchlorate ClO4-periodate IO4-permanganate MnO4-peroxide O2.
Acids and Review 1. Hypoiodite BrO2 1-chlorite CO4 2-phosphite PO5 3-percarbonate ClO1-bromate IO2 1-hyposulfite PO4 3-permanganate NO2 1-carbonite SO4 2-2. Na and NO 3 ÆNaNO 3 with two or more polyatomic ions has the polyatomic ions in parentheses.
Mg2 and 2NO 3 ÆMgNO 3 2 subscript 2 for charge balance. Percent composition in chemistry typically refers to the percent each element is of the compounds total mass. Hydrogen cyanide sometimes called prussic acid is a chemical compound with the chemical formula HCN.
H 2 PO 4. Iron III oxalate Fe2C2O43 20. 187 Occurrence Preparation and Properties of Nitrogen.
Similarly phosphite PO33- has one less oxygen than phosphate PO43-. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. 182 Occurrence and Preparation of the Representative Metals.
Since hydrogen sulfide H 2 S is a much stronger acid than water by more than ten million fold we expect and find thiols to be stronger acids than equivalent alcohols and phenols. 186 Occurrence Preparation and Properties of Carbonates. Tin IV carbonate.
The formula of the carbonate ion is ceCO_32-. Empirical Formula Hill Notation. If you bond H to an anion you add 1 to the charge that makes sense and add hydrogen to the name.
The basic equation mass of element mass of compound X 100. Hydrogen 1 H hydrogen 1 NH 4 ammonium 1 NH 4 ammonium 1 NH 4 ammonium 1 H hydrogen 1 H hydrogen 1 Pb lead IV plumbic 4 V vanadium V 5 vanadium As 5 arsenic 5 As arsenic 5 Cl chloride 1-F fluoride 1-O oxide 2-Cl chloride 1-Cl chloride 1-I iodide 1-I iodide I 1-iodide 1-Br bromide 1-Br bromide 1-Br bromide 1-O. The ammonium ion consists of one nitrogen atom and four hydrogen atoms.
The findings appeared within one hr and were completely reversible within 48 hr. The molecule is tetrahedral. Cadmium bisulfate CdHSO42 28.
Barium perchlorate BaClO42 21. It is a trivalent inorganic anion and a phosphite ion. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem.
It is a colorless liquid. Phosphite3- is a trivalent inorganic anion obtained by removal of all three protons from phosphorous acid. Name or write the formula for the following Type I polyatomic ionic compounds beryllium hydroxide BaIO32 sodium nitrite GaCNO3 ammonium chloride Ag2SO3 calcium bisulfate.
Formula Type Chemical Name FeO I Iron II oxide H 2 C 2 O 4 A Oxalic Acid Cu 3 PO 3 I Copper I phosphite GaC 2 H 3 O 2 3 I Gallium acetate H 2 CO 3 A Carbonic Acid CoCN 3 I Cobaltic cyanide N 2 O 3 Dinitrogen trioxideM SnCO 3 2 Stannic carbonateI MgClO 3 2 I Magnesium chlorate V 2 C 2 O 4 3 I Vanadium III oxalate P 2 O 5 M Diphosphorus pentoxide SrCN 2 strontium cyanideI KMnO 4. This page was. 184 Structure and General Properties of the Nonmetals.
Lead IV permanganate PbMnO44 26. If they begin with hydrogen name them as acids. For instance if you had a 800 g sample of a compound that was 200 g element X and 600 g element y then the percent composition of each element would be.
It is a reagent for generating other organophosphorus compounds exploiting the high reactivity of the P-H bond. Lithium phosphite Li3PO3 22. Periodate IO4- iodate IO3- iodite IO2- hypoiodite IO- But iodide is just I- 4.
You can buy it at most drug stores and use it for everything from disinfecting wounds to. It combines with many compounds to form crystalline solids useful as mild oxidizing agents.
/GettyImages-583679458-7ea6c76f50554eefb9a04054658237fa.jpg)
Hydrogen Peroxide Shelf Life And Effectiveness
Hydrogen peroxide decomposes into water and oxygen upon heating or in the presence of numerous substances particularly salts of such metals as iron copper manganese nickel or chromium.

Water and hydrogen peroxide. For water treatment concentrations of 35 or 50 hydrogen peroxide are used. It has physical properties that are very similar to water with the exception that it is 40 denser. The actual treatment procedure is simple.
Mix a paste of two thirds baking soda to one third hydrogen peroxide spread it over affected areas let it sit for 30 minutes and then rinse away. Hydrogen peroxide - urea also called Hyperol artizone urea hydrogen peroxide and UHP is a solid composed of equal amounts of hydrogen peroxide and ureaThis compound is a white crystalline solid which dissolves in water to give free hydrogen peroxide. Simply put hydrogen peroxide it is a mixture of water and oxygen.
In the case of hydrogen peroxide this is what makes it useful to remove soap scum and as a disinfectant. The brown bottle of 3 hydrogen peroxide from the drugstore or pharmacy is ideal for treating an abscess. Hydrogen peroxide solutions look like water and can be dissolved in water unrestrainedly.
The result is clean disinfected. In theory hydrogen peroxide could. 16 Fl Oz Pack of 1 46 out of 5 stars 1091.
Consult your doctor before trying these. The selective reduction of oxygen to water in such biological systems is crucial not only in order to maximize the energy produced for cellular metabolism but also because hydrogen peroxide is. It is usually stored with a stabilizer in an acid solution.
To make a gallon of 3 peroxide. It has antiseptic and antibacterial properties. Hydrogen peroxide - urea contains solid and water-free hydrogen peroxide which offers a higher stability and better controllability than.
Hydrogen peroxide is made up of hydrogen and oxygen. You can create a solution with a 1-1 ratio of water and vinegar or just use drops of hydrogen peroxide. Excellent yields very short reaction times mild reaction conditions and the avoidance of harsh reagents are the main advantages of this method.
Anhydrous hydrogen peroxide is a colourless syrupy liquid that decomposes into oxygen and water very easily. The most popular use of hydrogen peroxide for oral health is to remove tooth. Hydrogen peroxide is a chemical compound that combines hydrogen with oxygen.
Hydrogen peroxide contains a single oxygen-oxygen bond. Hydrogen peroxides antibacterial and antifungal qualities make it a great product for killing fungus and mold in the garden as well as in the house. Hydrogen peroxide is the water molecule with one extra oxygen atom.
Hydrogen Peroxide 12 Percent in Distilled Water with No Added Stabilizers. 1 part 35 hydrogen peroxide plus 11 parts water 3 hydrogen peroxide. At low temperatures it becomes solid.
A metering pump automatically inserts a tiny amount of hydrogen peroxide in front of the pressure tank whenever the well pump turns on. When its oxygen-oxygen bond breaks hydrogen peroxide decomposes into water and oxygen. Whitening with Hydrogen Peroxide.
The extra oxygen molecule oxidizes which is how peroxide gets its power says Dr. But more specifically it is a compound with an oxygen-oxygen single bond H2O2 it is a colorless liquid and has a bitter taste. Hydrogen peroxide in the presence of zirconium tetrachloride is a very efficient reagent for the direct oxidative conversion of thiols and disulfides into the corresponding sulfonyl chlorides with high purity through oxidative chlorination.
In its pure form hydrogen peroxide is a bluish liquid. When this happens it releases free radicals that. Three-percent hydrogen peroxide the concentration most commonly available at drug stores there are higher potencies for bleaching and industrial use though is.
It is a versatile solution with many uses depending on. Cleaning carpet stains with hydrogen peroxide is simple. At high concentrations these solutions give off an irritating acidic smell.
Let it sit then remove with a sponge or cloth. Follow with a mist of distilled white vinegar if necessary. Never use full strength food grade hydrogen peroxide internally or.
To kill the fungus thats getting to your plants mix 4 or 5 tablespoons of 3 hydrogen peroxide with 1 pint of water in. Put clean cloth over the area and apply pressure to pick up the liquid If any of the stain remains repeat the treatment. To make 3 hydrogen peroxide from 35 hydrogen peroxide the general mixing guideline is.
Hydrogen peroxide is water H2O with an extra oxygen molecule H2O2. Account Lists. Rinsing for 30 seconds to 1 minute is quite common however if at any point it feels like the solution is hurting your mouth spit it out.
A paste of baking soda and hydrogen peroxide cuts through soap scum and hard water stains on tubs and tiles. The back-washing carbon filter system is installed after the pressure tank and filters the water. Hello Select your address All Hello Sign in.
Hydrogen peroxide is inflammable. 99 125Fl Oz Get it Wed Dec 1 - Fri Dec 3. You can use this guideline with.
Hydrogen peroxide H2O2 is a chemical compound that is a combination of water and hydrogen. In a clean gallon container combine 1 and ¼ cups of 35 food grade hydrogen peroxide with 14 and ¾ cups of water. GreenFist Hydrogen Peroxide Ready to Use All Purpose Glass Carpet.
Skip to main contentus. Cleaning Carpet Stains with Hydrogen Peroxide. This extra oxygen makes the product an oxidizer which means it can break down other compounds.
Hydrogen peroxide has antiviral antibacterial antimicrobial and disinfectant activities. Swish around in your mouth for up to 1 minute. Spray or pour peroxide onto the stain.
H 2 O 2 hydrogen peroxide is a colorless liquid that is similar to water in several ways. Spit out the solution making sure not to swallow. Otherwise known as a peroxide bond this is incredibly weak and unstable.
Hydrogen peroxide is unstable by itself and it will decompose when exposed to light. Mix ½ cup water and ½ cup hydrogen peroxide. Use 3 hydrogen peroxide on stains.
The amount of hydrogen peroxide in the solution is expressed in weight percentage. The best-known of these is sodium perborate NaBO 2 H 2 O 2 3H 2 O or NaBO 3 4H 2 O used in laundry. Hydrogen peroxide is an inexpensive natural remedy for a tooth abscess.
Well Water Hydrogen Peroxide Injection With Optional Contact Tank for Disinfection. However the chemical behavior of hydrogen peroxide and water differs significantly. The store-bought products are clear as water.
It also means that hydrogen peroxide decomposes into oxygen and water which makes it an environmentally friendly cleaning. Hydrogen peroxide H 2 O 2 is one of the most important fundamental chemicals in the modern chemical engineering industry as well as energy.
1052 125anhydrous 1790 157solution Formula. 184 Structure and General Properties of the Nonmetals.

Hydrogen Fluoride Chemical Compound Britannica
Ammonia has a higher boiling.

Properties of hydrogen fluoride. 187 Occurrence Preparation and Properties of Nitrogen. Hydrogen fluoride is an industrial raw material used in the manufacture of products including refrigerants gasoline and. Send questions or comments to doi.
186 Occurrence Preparation and Properties of Carbonates. In water hydrogen bonding causes linkages in the water molecules which result in the boiling point of water is more than that of the other compounds. For example water melts at 000 C and boils at 9998 C.
Hydrogen is the chemical element with the symbol H and atomic number 1. The difficulty in handling the element and its toxic. The stoichiometric hydrogenoxygen mixture explodes at its contact with a catalyst flame or under the action of an electric spark.
H 2 O is a liquid whereas H 2 S H 2 Se and H 2 Te are all gases at ordinary temperature. Streng published a paper called The Chemical Properties of Dioxygen Difluoride Although the title may not be thrilling Strengs experiments certainly were. The reaction is so vigorous in nature that the hydrogen gas produced during the reaction catches fire.
Dioxygen difluoride is a terrifying chemical which also goes by the charming nickname FOOF because it is two fluorine atoms joined by two oxygen atoms. NIOSH REL TWA 3 ppm 25 mgm 3 C 6 ppm 5 mgm 3 15-minute OSHA PEL TWA 3 ppm See Appendix G. Alkali metals react with water to form hydroxides and hydrogen gas is released in the process.
Type or paste a DOI name into the text box. This makes it possible to organize combustion of hydrogen in place of. Sulfur is in group 16 of the periodic table the same as.
Like hydrofluoric acid. Hydrogen is the most abundant chemical substance in the universe constituting roughly 75 of all normal matter. The unusually high boiling point of hydrogen fluoride among the halogen acid is due to the existence of hydrogen bonding.
Hydrogen is widely seen as a future transport fuel In the short term hybrid electric vehicles have potential to increase the demand for base-load power from grid systems. This substance is used to make many everyday products including aluminum plastics refrigerants and high octane gasoline. 182 Occurrence and Preparation of the Representative Metals.
It is two and a half times heavier than air. It is used. He received the 1906 Nobel Prize for Chemistry for isolating fluorine.
As a result the large iodide anion gets polarized. Stars such as the Sun are. It becomes a liquid at 34 C 29 F.
Hydrogen fluoride is a colorless corrosive gas or liquid made up of a hydrogen atom and a fluorine atom. When hydrogen fluoride is dissolved in water it is called hydrofluoric acid. Electronegativity according to Pauling.
Hydrogen fluoride mixes readily with water forming hydrofluoric acid. At 18 for water and 16 for methane their physical properties are very different. Chlorine is a greenish yellow gas at room temperature and atmospheric pressure.
185 Occurrence Preparation and Compounds of Hydrogen. Hydroflouric acid hydrogen fluoride forms a special type of hydrogen bond called a symmetric hydrogen bond. The use of hydrogen in the production of transport fuels from crude oil is increasing rapidly.
At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2. In 1962 chemist AG. 1 ppm 082 mgm 3.
All the halides except lithium fluoride LiF readily dissolve in water. 1810-3 gcm-3 at 20C. Hydrogen fluoridehydrofluoric acid is used extensively in the extraction processing and refining of metals rock brick and oil.
F H 2 O. For all practical purposes they are considered the same chemical. In aqueous solution fluoride has a p K b value of 108.
Methane melts at -1825 C and boils at -1615 C. DOT ID Guide. It is an intermediate for many chemical reactions and syntheses.
Prepared from lithium hydroxide and hydrogen fluoride or by dissolving lithium carbonate in excess hydrogen fluoride evaporating to dryness and heating to red heat. That is the following equilibrium favours the left-hand side in water. Electronic shell He 2s 2 2p 5.
The Merck Index - An Encyclopedia of Chemicals Drugs and Biologicals. 0136 nm -1. It is therefore a weak base and tends to remain as the fluoride ion rather than generating a substantial amount of hydrogen fluoride.
Chlorine - chlorine - Physical and chemical properties. It has a choking smell and inhalation causes suffocation constriction of the chest tightness in the throat andafter severe exposureedema filling with fluid. Its reaction with fluorine to form hydrogen fluoride is accompanied by explosion even at low temperatures.
The most powerful intermolecular force influencing neutral uncharged molecules is the hydrogen bondIf we compare the boiling points of methane CH 4 -161ºC ammonia NH 3 -33ºC water H 2 O 100ºC and hydrogen fluoride HF 19ºC we see a greater variation for these similar sized molecules than expected from the data presented above for polar compounds. Hydrogenair mixtures with volumetric hydrogen content of 475 are inflammable. Hydrogen Bonding in Water vs Hydrogen Sulfide.
1 This neutralization reaction forms hydrogen fluoride HF the conjugate acid of fluoride. Anhydrous hydrogen fluoride Aqueous hydrogen fluoride HF-A Hydrofluoric acid CAS No. 183 Structure and General Properties of the Metalloids.
Hydrogen is the lightest element. Chemical properties of fluorine - Health effects of fluorine - Environmental effects of fluorine. It is colorless odorless non-toxic and highly combustible.
Your browser will take you to a Web page URL associated with that DOI name. Nuclear energy can be used to make hydrogen electrolytically and in the future high-temperature reactors are likely to be used. The isolation of fluorine was for a long time one of the chief unsolved problems in inorganic chemistry and it was not until 1886 that the French chemist Henri Moissan prepared the element by electrolyzing a solution of potassium hydrogen fluoride in hydrogen fluoride.
This bond is much stronger than a regular hydrogen bond and can be seen in these acids when they are kept at high pressure. When hydrogen is covalently bonded to a highly electronegative atom such as fluorine.
The dipole is shown by an arrow with a cross at one end. The hydrogen chloride molecule has a dipole two poles which consists of a pair of opposite charges separated from each other.
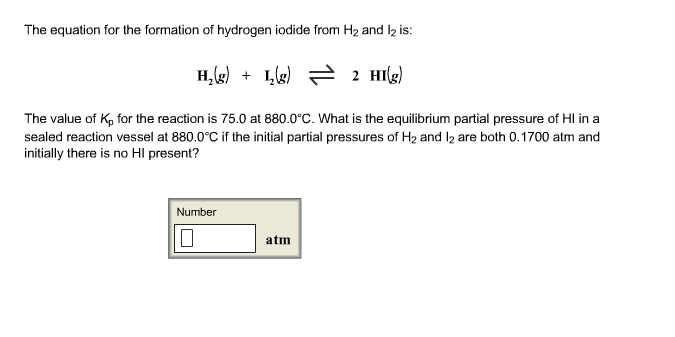
Solved The Equation For The Formation Of Hydrogen Iodide Chegg Com
Meanwhile the thyrocyte endoplasmic reticulum synthesizes two key proteins TPO and Tg.
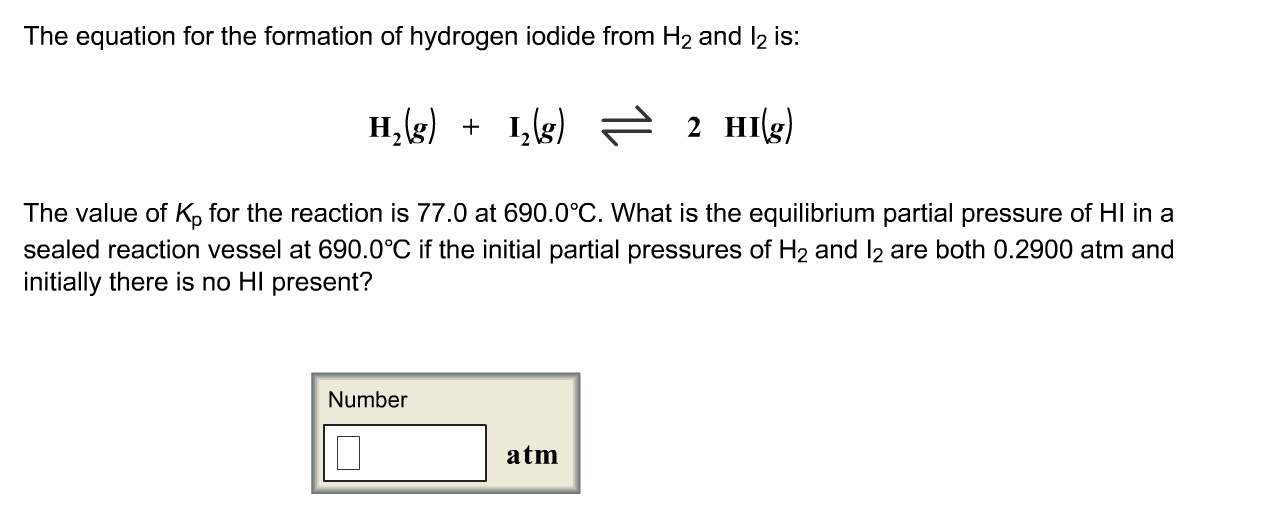
Formula of hydrogen iodide yields hydrogen iodide. Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction. It is present in teeth and bone and helps in metabolic processes. A metal plus a polyatomic ion yields an ionic compound.
For purposes of computing a formula mass it is helpful to rewrite the formula in the simpler format Al 2 S 3 O 12. Single or multiple bonds between carbon atoms are nonpolar. VIA O O2 oxide ion I I iodide ion S S2.
Formaldehyde and acetic acid have the same empirical formula CH 2 O. Silver iodide should be stored in cool dark areas away from the above materials. We will learn something about kinetics by experimentally determining a and b and by estimating k as a pseudo.
The cross is near the end of the molecule that is partially positive and the arrowhead is near the partially negative end of the molecule. The hydrogen peroxide produced subsequently oxidizes colorless iodide ion to yield brown iodine which may be visually detected. Before shipping silver iodide consult with the regulatory requirements of the US Department of Transportation.
Propene yields two products however only one predominates as. 1302g of C 8 H 17 OH 1-octanol formula mass 1302 amu 4549g of HgI 2 mercuryII iodide formula mass 4599 amu 320g of CH 3 OH methanol formula mass 320 amu and 2565g of S 8 sulfur formula mass. Explain the fact that in alkyl aryl ethers alkoxy group.
Salt with potassium iodide. Tg is a 660kDa glycoprotein secreted into the lumen of follicles whose tyrosyls serve as substrate for iodination and hormone formation. Thus two cysteine molecules combine into a less reactive molecule of cystine.
Molecular formulae indicate the simple numbers of each type of atom in a. Phosphoric Acid is an acid-containing four atoms of oxygen one atom of phosphorus and three atoms of hydrogen. D From alkenes i Addition of hydrogen halides.
It is also known as phosphoricV acid or orthophosphoric acid. To Learn about the structure of Acetic acid its preparations chemical physical properties uses and FAQs. The low reactivity of the disulfide bond protects skin from destruction by air water and in fact all the commonly occurring.
Clock-wise from the upper left. Ii directs the incoming substituents towards ortho and para positions in. TPO sits at the apical plasma membrane where it reduces H2O2 elevating the oxidation.
Potassium iodide given 12 and 24 hours before exposure yields a 90 and 70 protectant effect respectively. In its liquid form it appears as a clear colourless solution and in its solid form it. Write the equation of the reaction of hydrogen iodide with i1-propoxypropane iimethoxybenzene and iiibenzyl ethyl ether Ans.
However potassium iodide administered 1 and 3 hours after exposure results in an 85 and 50 protectant effect respectively. The first equation depicts the oxidation of glucose in the urine to yield glucolactone and hydrogen peroxide. The iodide reacts with magnesium to produce a Grignard product η 5-cpFeη 5-cp-MgI which reacts in a coupling reaction to yield diferrocene.
This product can also be produced by the reaction of iodine with the mercury compound shown earlier which yields the iodide. This is the actual chemical formula for formaldehyde but acetic acid has double the number of atoms. I activates the benzene ring towards electrophilic substitution.
H 3 O hydronium. Hydrogen iodide H I is a diatomic molecule and hydrogen halide. It allows the chemist to determine the amount of product that will form from a given amount of reactants or the amount of one reactant that is needed to react completely with some specific amount of the other reactant.
For small dry spills of silver iodide collect the material and deposit in sealed. Following the approach outlined above the formula mass for this compound is calculated as follows. Where k is the rate coefficient left I- right is the iodide concentration left H_2 O_2right is the hydrogen peroxide concentration and a and b are the order of the reaction with respect to iodide and hydrogen peroxide respectively.
Iodine can combine with sulfhydryl the sulfur-hydrogen bond of cysteine removing the hydrogen and by means of this oxidation setting the stage for sulfur atoms to bind to each other. An alkene is converted to corresponding alkyl halide by reaction with hydrogen chloride hydrogen bromide or hydrogen iodide. The formula for this compound indicates it contains Al 3 and SO 4 2 ions combined in a 23 ratio.
Acetic Acid CH3COOH- Acetic Acid is an organic compound with formula CH3COOHVinegar is a water solution of acetic acid containing 5-8 of acetic acid by volume. Although an enormous number of reactions can be carried out to produce. Potassium iodide administered more than 6 hours after exposure is thought to have a negligible protectant effect.
Some strips include an additional substance that reacts with iodine to produce a more distinct color change. An acid-base reaction is one in which a hydrogen ion H is transferred from one chemical species to anotherSuch reactions are of central importance to numerous natural and technological processes ranging from the chemical transformations that take place within cells and the lakes and oceans to the industrial-scale production of fertilizers pharmaceuticals and other. Go ahead and enjoy some dirty incest kinky fun with desi aunty sex.
It has a pungent smell and a sour taste. C 2 H 3 O 2-acetate OAc- C 2 O 4 2-oxalate. Formulas and Names of Some Polyatomic Ions NH 4 ammonium CO 3 2 carbonate H 3O hydronium HCO 3 hydrogen carbonate bicarbonate OH hydroxide OCN cyanate CN cyanide SCN thiocyanate O 2 2-2peroxide S 2O 3 thiosulfate N 3-azide CrO 4 2 chromate NO 2.
Formulas and Names of Some Polyatomic Ions. Phosphoric Acid is a weak acid with chemical formula H 3 PO 4. Visit BYJUS for more content.
Each sample contains 602 10 23 molecules or formula units100 mol of the compound or element. A metal plus a polyatomic ion yields an ionic compound. Intracellular iodide is then transported in the lumen of thyroid follicles.
Silver iodide may form explosive compounds with sodium potassium acetylene ammonia and hydrogen peroxide. Likewise the empirical formula for hydrogen peroxide H 2 O 2 is simply HO expressing the 11 ratio of component elements. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid a strong acidHydrogen iodide and hydroiodic acid are however different in that the former is a gas under standard conditions whereas the.
For this type of reaction to occur the reduction potential of the reactant receiving the electrons must be lower than the reduction potential of the reactant giving up electrons. Cadmium hydroxide CdOH 2 2510 14.

How To Write The Formula For Copper Ii Bicarbonate Youtube
The copper metal reacts with oxygen resulting in the formation of an outer layer of.
Copper hydrogen carbonate. MercuryI Hg 2 2. Copper is a chemical element with the symbol Cu from Latin. CaSO4 calcium sulfate 5.
Ionic bonds are atomic bonds created by the attraction of two differently charged ionsThe bond is typically between a metal and a non-metal. Calcium hydroxide CaOH 2 5. Cuprum and atomic number 29.
Copper will not react with hydrochloric acid. Hydrogen storage requires the reduction of an enormous volume of gas and an objective in hydrogen storage is to pack hydrogen as close as possible ie. FeC2H3O22 iron II acetate 4.
And blocks inositol. Answer 1 of 25. FeNO22 iron II nitrite 9.
Cu 2 OH 2. SnSO4 tin II sulfate 14. Cu2CO3 copper I carbonate 8.
At the same time fluctuations in the levels of iron and zinc which are essential elements were determined in the tissuesLevels of copper iron and zinc in blood were. Calcium hydrogen phosphate CaHPO 4 110 7. Hydroxide ions from say sodium hydroxide solution remove hydrogen ions from the water ligands attached to the copper ion.
Cu 2 OH. Cadmium sulfide CdS 810 28. If the atmosphere consists of high humidity moisture then this process is faster.
Water Hydrogen sulfate Hydrogen phosphate Hydrogen nitrate. This is our newest publication and has been created to support the school technician profession in Scotland. BaClO42 barium perchlorate 11.
Rats were fed a diet contain basic cupric carbonate at doses of 0 70 220 670 and 2000 ppm as cupric hydroxide and 12 months the levels of copper in the blood and tissues were determined by atomic absorption analysis. NO 2 Potassium. Ionic bonds also melt at high temperatures.
As a mineral it is known as tenoriteIt is a product of copper mining and the precursor to many other copper-containing products and chemical compounds. 1 Cs ion and 1 HCO 3-ion. Cadmium oxalate CdC 2 O 4 1510 8.
K2Cr2O7 potassium dichromate 6. Oxidation is a phenomenon whereby an element loses electrons andor hydrogen on interacting with another element. Copper sulphate blue stone blue vitriol are all common names for pentahydrated cupric sulphate CuSO 4 5H 2 O which is the best known and the most widely used of the copper salts.
ZnHCO32 zinc bicarbonate 15. Carbonate Iron II hydroxide Iron II sulfate Iron II phosphate Iron II nitrate Mg2Magnesium chloride Magnesium carbonate Magnesium hydroxide Magnesium sulfate Magnesium phosphate Magnesium nitrate HHydrogen chloride Hydrogen carbonate. CdOH2 cadmium hydroxide 13.
Indeed it is often the starting raw material for. The chemical element Calcium Ca atomic number 20 is the fifth element and the third most abundant metal in the earths crust. Calcium chromate CaCrO 4 7110 4.
It is a soft malleable and ductile metal with very high thermal and electrical conductivityA freshly exposed surface of pure copper has a pinkish-orange colorCopper is used as a conductor of heat and electricity as a building material and as a constituent of various metal alloys such as sterling. The formula unit for the ionic compound cesium hydrogen carbonate consists of which of the following. 2 Cs ions and 1 CO 3 2-ion.
Lithium Carbonate is the carbonate salt of lithium a soft alkali metal with antimanic and hematopoietic activities. What is the correct formula unit for the ionic compound copperII hydroxide. AgNO3 silver nitrate 10.
Uses of Copper Sulphate. To increase the density of hydrogen either work must be applied to compress the gas or the temperature must be decreased below the critical. H 3 O LeadII Pb 2.
H 3 O hydronium. Lithium interferes with transmembrane sodium exchange in nerve cells by affecting sodium potassium-stimulated adenosine triphosphatase Na K-ATPase. 2 Cs ions and 1 HCO 3 2-ion.
Why and why does this reaction even involve electron. Copper oxide is a base. Optical constants of Cu Copper Johnson and Christy 1972.
Calcium fluoride CaF 2 5310 9. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. 1 Cs 2 ion and 2 HCO 3-ions.
Alters the release of neurotransmitters. CopperI Cu 2 copperII Polyatomic positive ions often have common names ending with the suffix -onium. A black solid it is one of the two stable oxides of copper the other being Cu 2 O or copperI oxide cuprous oxide.
Carbonate CO3 2-chlorate ClO3-chlorite ClO2-chromate CrO4 2-cyanide CN-dichromate Cr2O7 2-hydrogen carbonate bicarbonate HCO3-hydrogen sulfate bisulfate HSO4-hydrogen phosphate biphosphate HPO4 2-hydroxide OH-hypochlorite ClO- iodate IO3-nitrate. H aq OH-aq H 2 Ol Pure water is neutral. Calcium carbonate CaCO 3 2810 9.
Once a hydrogen ion has been removed from two of the water molecules you are left with a complex with no charge - a neutral complex. NH 4 ammonium. The metal is trimorphic harder than sodium but softer than aluminiumA well as beryllium and aluminium and unlike the alkaline metals it doesnt cause skin-burns.
It is less chemically reactive than alkaline metals and than the other alkaline-earth. The structure of the bond is rigid strong and often crystalline and solid. 1 Cs 2 ion and 1 CO 3 2-ion.
Similar to iron and aluminum the element copper undergoes the process of oxidation if it is exposed to air. Cu H 2 O 2 CuO H 2 O CuO 2HCl CuCl 2 H 2 O Uses. KHCO3 potassium bicarbonate 7.
Affects cyclic adenosine monophosphate concentrations. Hydrogen carbonate HCO 3 1 sulfite SO 2 2 lithium Li 1 bisulfate hydrogen sulfate HSO 4 1 sulfide S 2 2 nickelII nickelous Ni 2 2 bromate BrO 3 1 thiocyanate SCN 1 potassium K 1 bromide Br 1 thiosulfate S 2 O 3 2 2 A note. In acid-alkali neutralisation reactions hydrogen ions from the acid react with hydroxide ions from the alkali.
Cadmium carbonate CdCO 3 5210 12. TinIV Sn 4. CopperII oxide or cupric oxide is an inorganic compound with the formula CuO.
PbCO32 lead II carbonate 12. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. Negative ions that consist of a single atom are named by adding the suffix -ide to the stem of the name of the element.
To reach the highest volumetric density using as little additional material and energy as possible. Cu2CO3 copper I carbonate 3. It can also be made by reacting copperII hydroxide copperII oxide or copperII carbonate with hydrochloric acid and from pure copper and from 11 solution of hydrogen peroxide and hydrochloric acid where copper first get oxidized to CuO from H2O2 and then reacts with HCl to form CuCl2 reaction goes like this.