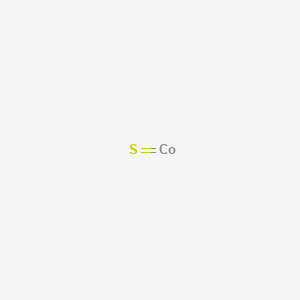Copy this to my account. C 25 H 42 N 7 O 17 P 3 S xLi CAS No.

Zinc Sulfide 99 99 Trace Metal Basis 10 Microns Acros Organics Other Inorganic Compounds Chemicals Fisher Scientific
In an experiment a mixture of copper and sulfur was heated to produce a sample of copper sulfide.

Zinc sulfide formula. 3P zinc carbide Zn 2C manganese IV sulfide MnS 2 cobalt II bromide CoBr 2 manganese II carbide Mn 2C phosphorus V nitride P 3N 5 gold I iodide AuI nickel III phosphide NiP iron II bromide FeBr 2 copper II sulfide CuS aluminum sulfide Aℓ 2S 3 silicon iodide SiI 4 lead IV carbide PbC aluminum fluoride AℓF 3 arsenic V nitride As 3N 5 mercury I phosphide Hg 3P cobalt III. 63 Cu 2 S surface exhibits higher adsorption energies than CuS surface which indicates higher role of Cu 2 S or non-stoichiometric Cu x S phases in the adhesion between rubber and brass. Chemical Formulas And Names Of Ionic Compounds.
The obtained ZnIn2S4 catalyst can reduce CO2 to. The iron content is normally less than 25 by weight. NaMnO4 sodium permanganate 50.
It is on the World Health Organizations List of Essential Medicines a list of the most important medication needed in a basic health system. Hg2Cl2 mercury I chloride 57. Chemical Formula Nomenclature Practice.
Both elements exhibit only one normal oxidation state 2 and the Zn2 and Mg2 ions are of similar size. Zinc has many commercial uses as coatings to prevent rust in dry cell batteries and mixed with other metals to make alloys like brass and bronze. 83762 free acid basis Compare Product No.
Use the stock form for the transition metals. E-mail to a friend. 71 silicon dioxide SiO 2 72 nickel III sulfide Ni 2 S 3 73 manganese II phosphate Mn 3 PO 4 2 74 silver acetate AgC 2 H 3 O 2 75 diboron tetrabromide B 2.
Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Although this mineral is usually black because of various impurities the pure material is white and it is widely used as a pigment. You should complete this by Sunday.
Zinc nitrite 70 V 2 S 3 vanadium III sulfide Write the formulas for the following chemical compounds. The chemical formula of sphalerite is ZnFeS. Redness irritation burning sensations dry hair and blistering if the shampoo is left on the skin for too long.
AMPAIRE TALENTS March 22 2020. And 2 deposition from aqueous brine solutions at temperatures in the 300600 C 5721112 F range and at relatively high pressure such as at the seafloor or several kilometres. Zinc iron II iron III gallium silver lead IV chloride ZnCl 2.
63 Zinc sulfide seems to prefer adsorption via sulfur-containing groups whereas preferable adsorption on. NaCIO4 sodium perchlorate 47. Formula writing rules to write the correct chemical formulas for each compound.
Give the formula for the following. Importantly selenium sulfide shampoos have been known to discolor dyed hair and can discolor metallic jewelry so should be removed. Sphalerite often contains trace to minor.
Sphalerite is a sulfide mineral with the chemical formula ZnFeS and an ore of zinc. Ionic Compounds Naming and Formula Writing. 1 NaF is sodium fluoride 2 K2CO 3 is potassium carbonate 3 MgCl 2 is magnesium chloride 4 BeOH 2 is beryllium hydroxide 5 SrS is strontium sulfide 6 Cu 2S is copper I sulfide 7 ZnI 2 is zinc iodide 8 Ca 3PO 42 is calcium phosphate 9 NH 4I is ammonium iodide 10 MnNO 33 is manganese III nitrate.
Ionic Compounds Naming and Formula Writing. Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. When the iron content is high sphalerite is an opaque black variety called marmatite.
Here we report an indium sulfide catalyst that is stabilized by adding zinc in the structure and shows dramatically improved stability. Zinc sulfide or zinc sulphide is an inorganic compound with the chemical formula of ZnS. It is found in air soil and water and is present in all foodsPure zinc is a bluish-white shiny metal.
Zinc and copper sulfide surfaces are formed in adhesive interlayer during rubber vulcanization. Solubility Product Constants near 25 C. Copy this to my account.
The names are found by finding the intersection between the cations and anions. KCIO potassium hypochlorite 48. Complete these in lab and on your own time for practice.
Compound Name Type of Compound. Harrys also offers a regular Anti-Dandruff formula with 1 percent zinc pyrithione Not only does this sulfate-free shampoo relieve itching and flaking it only costs 7 for a sizable 14. Compound Interest Formula Online.
In some respects zinc is chemically similar to magnesium. Al2S3 aluminum sulfide 54. Zinc is one of the most common elements in the earths crust.
ZnCH3COO2 zinc acetate 58. Sodium thiosulfate ____Na2S2O3_____ 3. PbO2 lead IV oxide 56.
The chemical symbol for Zinc is Zn. ZnBr2 zinc bromide 45. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
Centroid Of Trapezoid Formula. Mg3N2 magnesium nitride 49. Fe2CrO43 iron III chromate 46.
Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound. The amount of iron substitution that occurs depends upon iron availability and temperature with higher temperatures favoring higher iron content.
Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box. Sulfur dioxide ____SO2_____ 2. Mean And Standard Deviation Formula.
Formula to name problems. Zinc sulfate is the inorganic compound with the formula ZnSO4 and historically known as white vitriol. Ionic Compound Formula K sp.
Calculate the empirical formula of copper sulfide. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3.
1 separation of an immiscible sulfide melt during the early stages of crystallization of basic magmas. Sulfide mineral deposits originate in two principal processes both of which have reducing conditions. German geologist Ernst Friedrich Glocker discovered sphalerite in 1847 naming it based on the Greek word sphaleros meaning deceiving due to the difficulty of identifying the mineral.
This is the main form of zinc found in nature where it mainly occurs as the mineral sphalerite. A zinc and copper alloy is used to make pennies in the United States. K2SO4 potassium sulfate 59.
E-mail to a friend. Selenium sulfide shampoos are associated with a higher incidence of adverse effects than ketoconazole with the most common reactions being. Empirical Formula Hill Notation.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. In its dense synthetic form zinc sulfide can be transparent and it is. Perimeter Of Rectangle Formula.
It is a zinc sulfide containing variable amounts of iron that substitutes for zinc in the mineral lattice. The following data were obtained. SnSO42 tin IV sulfate 55.
Hydrogen sulfide can also result from industrial activities such as food processing coke ovens kraft paper mills tanneries and petroleum refineriesHydrogen sulfide is a flammable colorless gas with a characteristic odor of rotten eggs. Chemical Formula Nomenclature Practice.
CobaltII hydroxide CoOH 2 1610 15.
Cobalt sulfide formula. Use the stock form for the transition metals. We had an interest in indium sulfide as a catalyst because S-doped In was shown by Wang and co-workers to. Sodium thiosulfate ____Na2S2O3_____ 3.
CopperI cyanide CuCN 3210 20. Copy this to my account. CopperI chloride CuCl 1210 6.
E-mail to a friend. To further improve its energy density and application high-voltage LiNi 05 Mn 15 O 4 LNMO spinel cathode is highly desirable due to its high energy density low cost environmental friendliness and Co-free nature. CobaltII sulfide CoS 410 21.
Na Mg 2 Non. Show all questions. CopperII arsenate Cu 3 AsO 4 2 7610 36.
KMnO4 potassium permanganate Write the chemical formula for each of the following ionic compounds. Manganese II carbonate MnCO3 73. CopperI iodide CuI 1110 12.
Zinc iron II iron III gallium silver lead IV chloride ZnCl 2. Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution. B The atoms in lithium sulfide are held together by bonds C.
Metals lose electrons to produce positve ions called cations. It is a red crystalline solid. Which statement about lithium sulfide is correct1 point A The chemical formula for lithium sulfide is LiS2.
Potassium bromide KBr 62. Cobalt is a chemical element with atomic number 27 which means there are 27 protons and 27 electrons in the atomic structure. B The atoms in lithium sulfide are held together by bonds C.
The names are found by finding the intersection between the cations and anions. The free element produced by reductive smelting is a hard lustrous silver-grey metal. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Like nickel cobalt is found in the Earths crust only in a chemically combined form save for small deposits found in alloys of natural meteoric iron. Copy this to my account.
8 water molecules octahydrate. Give the formula for the following. Barium sulfide BaS 72.
The chemical symbol for Cobalt is Co. Ionic Compounds Naming and Formula Writing. 13 potassium fluoride is KF 14 ammonium sulfate is NH 42SO 4.
Sodium chloride NaCl and magnesium oxide MgO. The compound forms several hydrates CoCl 2 n H 2 O for n 1 2 6 and 9. Ca2 Calcium S2-Sulfide Sr2 Strontium Se2-Selenide Ba2 Barium Zn2 Zinc Cd2 Cadmium 3 Al3 Aluminum 3- N3- Nitride P3- Phosphide Table 2 Metals That Form More Than One Monatomic Ion ELEMENT ION FORMULA SYSTEMATIC NAME COMMON NAME Chromium Cr2 Chromium II Chromous Cr3 Chromium III Chromic.
Tin IV sulfide 34 TiCN 4 titanium IV cyanide 35 KMnO 4 potassium permanganate 36 Pb 3 N 2 lead II nitride 37 CoCO 3 cobalt II carbonate 38 CdSO 3 cadmium sulfite 39 CuNO 2 2 copper II nitrite 40 FeHCO 3 2 iron II bicarbonate Name the following chemical compounds. Claims of the formation of tri- and tetrahydrates have not been confirmed. Manganese IV sulfide MnS 2 cobalt II bromide CoBr 2 manganese II carbide Mn 2C phosphorus V nitride P 3N 5 gold I iodide AuI nickel III phosphide NiP iron II bromide FeBr 2 copper II sulfide CuS aluminum sulfide Aℓ 2S 3 silicon iodide SiI 4 lead IV carbide PbC aluminum fluoride AℓF 3 arsenic V nitride.
Cobalt is found in the Earths crust only in chemically combined form save for small deposits found in alloys of natural meteoric iron. Barium oxide BaO lithium sulfide Li 2S magnesium bromide MgBr 2. Ionic Compound Naming and Formula Writing List 1.
It is usually supplied as the hexahydrate CoCl 2 6 H 2 O which is. The free element produced by reductive. Which statement about lithium sulfide is correct1 point A The chemical formula for lithium sulfide is LiS2.
The transfer of electrons between metals and non-metals produces charged particles called ions. When the chemical formula for a hydrated ionic compound is written the formula for the ionic compound is separated from the waters of hydration by a centered dot. 2 In a molecule of lithium sulfide there are two atoms of lithium and one atom of sulfur.
An ionic compound is composed of a metal and a non-metal. Complete these in lab and on your own time for practice. Co2SO43 cobalt III sulfate 60.
Synthesis and characterizations of catalysts. NH42S ammonium sulfide K3PO4 potassium phosphate ZnNO32 zinc nitrate Fe2SO43 ironIII sulfate or ferric sulfate CuCO3 copperII carbonate or cupric carbonate Note. In a formula parentheses are used around a polyatomic ion only when there are 2 or more of that polyatomic ion in a formula unit ie when the subscript is not 1.
41 lithium acetate LiC 2 H 3 O 2 42 iron II phosphate. You should complete this by Sunday. Sulfide all-solid-state lithium-ion batteries represent one of the most promising energy storage technologies duo to higher safety and ionic conductivities.
CobaltII carbonate CoCO 3 1410 13. CobaltII chloride is an inorganic compound of cobalt and chlorine with the formula CoCl 2. E-mail to a friend.
Cobalt-based blue pigments cobalt blue have been used since ancient times for. 5 SrS is strontium sulfide 6 Cu 2S is copper I sulfide 7 ZnI 2 is zinc iodide 8 Ca 3PO 42 is calcium phosphate 9 NH 4I is ammonium iodide 10 MnNO 33 is manganese III nitrate 11 FePO 4 is iron III phosphate 12 CoCO 3 is cobalt II carbonate Name to formula problems. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator.
Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound.
2 In a molecule of lithium sulfide there are two atoms of lithium and one atom of sulfur. The dihydrate is purple and hexahydrate is pink. Formula writing rules to write the correct chemical formulas for each compound.
CobaltIII hydroxide CoOH 3 1610 44. Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. Cobalt is a chemical element with the symbol Co and atomic number 27.
Sulfur dioxide ____SO2_____ 2. People can smell it at low levels. It is commonly known as hydrosulfuric acid sewer gas and stink damp.
It was designed by Alfred Stock 1876-1946 a German chemist and first published in 1919. Greek prefixes are attached to the word hydrate to indicate the number of water molecules per formula unit for the compound eg BaOH 2 8H 2 O. Compound Name Type of Compound.
History- The type of naming you will learn about is called the Stock system or Stocks system.
3D left and center and 2D. 36 Full PDFs related to this paper.
UOP202-14 Disulfide Sulfur in Light Petroleum Distillates and LPG.

Molecular mass of sodium sulfide. METHOD 6013 Issue 1 dated 15 August 1994 - Page 2 of 4 SAMPLING. The model should show 27 protons and 32 neutrons B. The model should show 59 protons and 27 neutrons.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Determine the molar mass of sodium oxide Na 2 O.
Which uses a molecular sieve sampler but has poor stability. Molecular mass of H 2 O 2 x atomic mass of H 1 x atomic mass of O 2 x 100797 1 x 159994 amu 1802 amu. An organic compound is known to be composed of 4660 carbon 6800 hydrogen and 4660 oxygen.
Yes because the chemical factor 15921271 is just a bit greater than unity indicating that the mass of the product will slightly exceed that of the copper. This effect could also be demonstrated by preincubating cells for 3-20 min within 5-10 mM sodium hydrosulfide. The molecularformula mass is numerically equal to the mass of one mole of the substance.
Production of hydrogen sulfide can be detected when ferrous sulfide a black precipitate is produced as a result of ferrous ammonium sulfate reacting with H2S gas. The atomic mass of chlorine is 3545 g per mole while the atomic mass of sodium is 2299 g per. Sodium sulfide anhydrous is a yellow to brick red crystalline mass or fused solid with an odor of rotten eggs.
NIOSH Manual of Analytical Methods NMAM Fourth Edition 81594. A student wants to create a model of a cobalt atom. Sodium bicarbonate baking soda NaHCO3 can be purified by dissolving it in hot water 60 C filtering toexample.
As an example if you need to do figure out the molar mass of NaCl youll have to find the atomic mass of both sodium and chlorine. Determine the molar mass of ozone. For network solids the term formula unit is used in stoichiometric calculations.
The molecular mass can be calculated from the chemical formula and is expressed in conventional atomic mass units equal to 112 of the mass of a neutral carbon-12 12 C isotope atom. Is this answer reasonable. Doing this should give you the molar mass of a compound which is comprised of various kinds of atoms.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. Patch clamp studies of neuroblastoma cells have shown that in the presence of sodium hydrogen sulfide the in vitro precursor of hydrogen sulfide addition of the sulfonated amino acids taurine or cysteic acid resulted in reversible abolition of the inward of the sodium currents.
1 mole molar mass could be atomic mass from periodic table or molecular mass 1 mole 224 L of a gas at STP You do not need to worry about this yet Each definition can be written as a set of two conversion factors. The relations among amount of substance in. Substance MW or FW molar mass Fe 55.
Type 13X offers enhanced adsorption performance over the type A zeolite and it can remove impurities too large to fit into. 00 amu 3 The molecular mass of H2SO4 98. 1 mole molar massg can be written as ____1 mole OR _molar mass g.
H 3 x 1. Sodium and aluminium are taken to compare. UOP212-05 Hydrogen Sulfide Mercaptan Sulfur and Carbonyl Sulfide in Hydrocarbon Gases by Potentiometric Titration.
We would like to show you a description here but the site wont allow us. Determine the molar mass of calcium sulfide CaS. 1 Cobalt has a mass number of 59 and an atomic number of 27.
The mass of copper sulfide formed will be determined by the mass of copper available. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. Ammonium hydroxide solution 25.
The model should show 27 protons and 27 neutrons. A Laboratory Manual Third Edition 1982. If exposed to moist air it is liable to spontaneous heating and may cause ignition of nearby combustible material.
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Which statement about the model is correct1 point A. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
Beef extract 30 g Peptone 300 g Ferrous ammonium sulphat 02 g Sodium thiosulphate 0025 g Agar 30g Final pH at 25C 7302 Distilled water 1000ml. UOP209-00 Alkalinity Sulfide and Mercaptide Analyses of Used Refinery Caustic Solutions. So melting point of aluminium is greater than sodium.
Sodium loses its outer electron to give it a stable electron configuration. What is its empirical formula. CO3- because the background.
Molecular mass - when molecular mass increase. Molar mass has units of grams per mole gmol. 4 To obtain one mole of copper atoms 602 x 10 23 atoms weigh out 6355 g copper.
Sodium is s block metal and aluminium is a p block metal. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. Due to release of three electrons and less radius metallic lattice of aluminium is much stronger than sodium.
Hydrogen peroxide 30 3. How would you calculate the empirical formula of acetic acid CH3COOH. It also has the highest theoretical capacity of the common adsorbents and excellent mass transfer rates.
UOP163-10 Hydrogen Sulfide and Mercaptan Sulfur in Liquid Hydrocarbons by Potentiometric Titration. 15 g Cu 1592 g Cu 2 S 1271 g Cu 188 g Cu 2 S Check. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
5 The molar mass M of a substance is the mass of one mole of entities atoms molecules or formula units of the substance. HYDROGEN SULFIDE 6013 H 2S MW. Why d block elements.
Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. But both are located in 3rd period of the periodic table. For example the molecular weight of water would be obtained by the following process.
The relation between molecular formula mass and molar mass. A short summary of this paper. Full PDF Package Download Full PDF Package.
Copper will react with sulfur to form a copper sulfide if 1500g copper reacts with sulfur to form 1880g of copper sulfide what is the empirical formula of the copper sulfide. Science CHECK MY ANSWERS. The 13X molecular sieve is the sodium form of zeolite X and has a much larger pore opening than the type A crystals with an effective pore diameter of 10 angstroms.
A Laboratory Manual Third Edition. A Laboratory Manual Third Edition.