The 3-D structure of the protein determines which surface residues will be available to contact the columns stationary phase. Propionaldehyde is made by hydroformylation ethylene.
1 Propanol N Propyl Alcohol Ar 201072500 Reagents 71 23 8 Biochemopharma
The complete combustion of methanol is as follows.

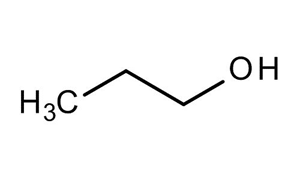
N-propanol structure. IUPAC Name propan-1-ol. These alcohols form hydrogen bond with water due to the polar OH functional group. Concentrated alcohols mainly ethanol 1-propanol called also n-propanol and 2-propanol called isopropanol and mixtures thereof.
2 4 6 Alcohol-free products are generally based on disinfectants such as benzalkonium chloride BAC or on antimicrobial agents. It is a short-chain primary fatty alcohol and a member of propan-1-ols. CATEGORY 1A Sampling and Measurement of noise illumination and heat.
Sample pI is a guideline and not absolute. Oxidation of Propanol CH 3 CH 2 CH 2 OH with PCC and KMnO 4. Propan-1-ol is the parent member of the class of propan-1-ols that is propane in which a hydrogen of one of the methyl groups is replaced by a hydroxy group.
The higher-order alcohol n-propanol is desired for its high volumetric energy density 27 MJ l 1 ref. 1 6 At those concentrations alcohol immediately denatures proteins effectively neutralizing certain types of microorganisms. The net charge determines the form of IEC anion exchange or cation exchange to be applied.
This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. 1-Propanol is a primary alcohol with the formula CH 3 CH 2 CH 2 OH and sometimes represented as PrOH or n-PrOHIt is a colorless liquid and an isomer of 2-propanolIt is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry mainly for resins and cellulose esters and sometimes as a disinfecting agent. As the number of carbons per polar functional group increase solubility decreases.
Valid until September 27 2022. Prices Kandla Mumbai Mundhra Markets covered. Propan-1-ol n-propyl alcohol 1-propyl alcohol or n-propanol are all names for this colourless oil.
Keeping in mind the relative molecular weights of the compounds you can see there is a decreasing effect of the hydrogen bonding and other effects on the n-alcohol series as we move to larger chains. Further 2-phenoxyethanol and 1- and 2-phenoxypropanols are used phenolic substances such as phenol also called carbolic acid cresols such as thymol halogenated chlorinated brominated phenols such as hexachlorophene triclosan trichlorophenol. Please note that Hydrophilic PVDF syringe filters are not compatible with majority of strong acids and caustic solutions such as dimethyl sulfoxide dimethylformamide DMF acetone ketones esters and ethers.
Our PVDF filters are high defined pore structure low nonspecific binding compatible with organic and aqueous solvents. Propionaldehyde is catalytically hydrogenated to produce 1-propanol. NSC 68472 Permanent link for this species.
1-Phenyl-1-propanol C9H12O CID 7147 - structure chemical names physical and chemical properties classification patents literature biological activities. 2CH3OHl3O2g2CO2g4H2Ol Which of the following statements is true regarding the. Alcohol-based products typically contain between 60 and 95 percent alcohol usually in the form of ethanol isopropanol or n-propanol.
Ethyl and isopropyl alcohol are similar molecularly but have different chemical structures. The surface structure of the copper electrodes strongly depends on the pre-treatment 35 and reconstructions take place under the CO 2 RR 363738Figure 1 and. The distinct lattice fringe of 0270 nm corresponds to the 221 crystallographic plane of GaFeO 3 phase.
Which of the following classes of alcohols will oxidize under mild conditions to form a ketone. Calculate the heats of combustion of these alcohols. Compatible with organic and aqueous samples.
Academiaedu is a platform for academics to share research papers. Also propanol can be oxidized to propanal by mild oxidation agents such as PCC. Data obtained from the NIST Webbook.
Cation exchange is used at pHs below a proteins pI while anion exchange is used at pHs above a proteins pI. When 100 g of each of these alcohols is burned in air brat is liberated as indicated. The solvent properties of isopropyl alcohol is similar to ethyl alcohol.
COMPANY NAME ACCREDITATION NUMBER VALIDITY CATEGORY ADDRESS CONTACT DETAILS. 2i-l displayed the selected SEM image of GaFeO 3 and the EDS elemental mapping images of Ga Fe and O. Propanol 1-propanol n-propanol is a primary alcohol which can be oxidized to propanoic acid by using strong oxidizing agents.
The solubility of alcohols with four to five carbons is given. Organic compounds with one polar functional group and a low number of carbon atoms such as methanol ethanol and n-propanol are highly soluble miscible in water. Fill in the missing atoms or groups to complete the structure of the more complete oxidation of n-propanol below.
H O OH H. Low-density polyethylene LDPE films coated with a mixture of polyamide resin in i-propanoln-propanol and a bacteriocin solution showed an antimicrobial activity against Micrococcus flavus. Propanol is one of the most common types of alcohol.
9cost-effective renewable propanol would offer a sustainable liquid fuel for existing. Isopropyl alcohol is widely employed in solvent applications. The internal loose structure of the hierarchical GaFeO 3 may enable the material desirable gas sensing performance by virtue of excellent gas diffusion and fast mass transportation.
In chemistry the definition of alcohol is an organic molecule that contains a hydroxyl group bonded to. Methanol ethanol and n-propanol are three common alcohols. A must-have and indispensable service for individuals and organizations associated with chemical markets across the.
This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Propanol has the formula CH 3 CH 2 CH 2 OH. We would like to show you a description here but the site wont allow us.
Use this link for bookmarking this species for. The incorporation of 10 ww potassium sorbate in low-density polyethylene films lowered the growth rate and maximum growth of yeast and lengthened the lag period before mould growth Han and Floras. Isopropyl alcohol was one of the first petrochemical products to be manufactured and produced since 1920.
Isopropyl alcohol commonly referred to as Isopropanol or n-propanol or dimethylcarbinol is a colourless and flammable liquid with the formula C 3 H 8 O. It has a role as a protic solvent and a metabolite.
High boiling esters are used as softeners plasticizers. Ships Today 387 Product Category.

Isobutyl Acetate Natural 97 Fcc Fg 110 19 0
Salts 63 stable isotopes 48 kits 31 buffers 29 building blocks 29 solvents 29 Show More.

Isobutyl acetate structure. 001 - dihydromyrcenol 005 - benzyl propionate 005 - linalyl acetate 005 - phenethyl alcohol 001 - aldehyde C-14 10 020 - amyl cinnamaldehyde 001 - mahy shiff 005 - naphthyl ethyl ether 001 - aldehyde C-16 10 005 - geraniol an antimicrobial antiseptic and disinfectant that is used also as an aromatic essence and preservative in pharmaceutics and perfumery. Odor detection thesholds Odour. In order to.
17000 μgcapitaday Maximised Survey-derived Daily Intakes MSDI-USA. Ethyl acetate itself is a colourless liquid at room temperature with a pleasant fruity smell bp. If irritation or pain persists see a doctor.
This colourless liquid a ketone is used as a solvent for gums resins paints varnishes lacquers and nitrocellulose. Structure-guided optimization of 1H-imidazole-2-carboxylic acid. Pregelatinized Starch and Corn Starch.
Methacrylic Acid and. The inhibitory activity was likely improved with increase in the size of the alkyl R 1 groups. 7 Chemical Name Chemical Abstract Service CAS Number.
Share and shareholder structure Bond and convertible Voting rights Directors dealings Corporate governance Annual General Meeting Downloads IR contact Share price Read more. Use levels for FEMA GRAS flavoring substances on which the FEMA. Ethyl acetate has many uses.
Methyl isobutyl ketone MIBK is a solvent used in numerous products and processes and may be present in the air of the workplace as a vapor. Ethyl acetate is used to extract organic solutes from aqueous solutionsfor example to remove caffeine from coffee. Rules for alkane nomenclature Find and name the longest carbon chain Name the groups attached to the longest carbon chain Number the chain consecutively starting at the end nearest a substituted group Designate the.
Melting Point C Physical Form. Ethyl Acetate Methyl Acetate. Of fresh liver of the acetone powder per ml.
Aromatic solvents feature a benzene group cyclic structure of 6 carbons. It is a primary alcohol and an alkyl alcohol. In chemistry an ester is a chemical compound derived from an acid organic or inorganic in which at least one OH hydroxyl group is replaced by an Oalkyl group.
055 044 Sustainability Back Sustainability. It derives from a hydride of an isobutane. Our approach Footprint Innovation Sourcing Care Reports Policies Standards Audits Trending now Read more.
Esters are structurally derived from carboxylic acids by replacing the acidic hydrogen by an alkyl or aryl group. Recommendation for styralyl acetate usage levels up to. Class Acetic acid Ethanoic acid CH 3 COOH Calss 3 Acetone 2-Propanone CH 3 COCH 3.
Products Building Blocks Explorer Technical Documents Site Content Papers Genes. Cellulose nitrate is dissolved in ethyl acetate and butyl acetate to form lacquers. P302 P352.
It has a role as a Saccharomyces cerevisiae metabolite. Ethyl acetate 51 18 31 Trimethyl phosphate 39 37 24 Diethyl carbonate 64 12 24 Diethyl sulfate 42 39 19 37 n-Butyl acetate 60 13 27 Isobutyl acetate 60 i 5 25 38 Isobutyl isobutyrate 63 12 25 39 Isoamyl acetate 60 12 28 40 Cellosolve acetate 51 i 5 34 Ethyl lactate 44 21 35 Butyl lactate. FTIR showed there were copious hydroxyl groups on the surface of d-MnO 2 for the adsorption.
Chemistry Magazine Online gives you another post with interesting chemistry news articlesAbstracts d- MnO 2 was prepared with hydrothermal treatment and its crystalline structure was verified with X-ray diffraction spectroscopy. An antiadipogenic activity of isolated bmGAGs was also investigated and results indicate that these bmGAGs exhibited potential anti-adipogenic effect and. The adsorption capacity of three kinds of heavy metal ions Pb.
Amyl Propyl Butyle Acetate. The catalytic performance of 2 was evaluated in the model reaction of cis-cyclooctene Cy8 with tert-butyl hydroperoxide TBHP at 70 C using different types of solvents namely ααα-trifluorotoluene TFT and the bio-based solvents ethanol EtOH ethyl acetate EA and isobutyl acetate IBA. Isobutyl acetate Acetic acid isobutyl ester CH 3.
Millipore 5 Sigma-Aldrich 697 Supelco 9 Boiling Point C Feature. Mar 19 2012 Other than methanol we can add other additives such as isopropyl alcohol acetone methyl ethyl ketone methyl isobutyl ketone and denatonium to make denatured alcohol. We would like to show you a description here but the site wont allow us.
In conclusion the composition and structure of GAGs from the bovine milk was characterized by cellulose acetate electrophoresis disaccharide composition and found that the bmGAGs composed majorly of chondroitindermatan sulfate. Ethyl Acetate a fast evaporation solvent widely used in many fast drying systems. 50000 in the fragrance concentrate.
Official IUPAC naming recommendations are not always followed in practice and the common or trivial name may be used. Ethyl acetate structure shown above is the most familiar ester to many chemistry students and possibly the ester with the widest range of uses. Magnesium Carbonate direct compression 45 Magnesium Oxide.
In a 24 h batch run 91100 conversion was reached for all solvents and cyclooctene. Methyl isobutyl ketone MIBK is the common name for the organic compound 4-methylpentan-2-one condensed chemical formula CH 3 2 CHCH 2 COCH 3. The solvent evaporates as the lacquer dries leaving a thin film on the surface.
Glycerides which are fatty acid esters of glycerol are important esters in biology being one of the main classes of lipids and. It provides an unambiguous structure. Stearoyl-CoA Desaturase 1 Inhibitor MF-438 CAS 921605-87-0 is a cell-permeable inhibitor of Stearoyl-CoA Desaturase 1 SCD1.
IC50 of 23 nM. It also is used to remove nail polish and paint. 1800 μgpersonday Structure Class.
Maximised Survey-derived Daily Intakes MSDI-EU. The American Conference of Governmental Industrial Hygienists ACGIH threshold limit value-time-weighted average TLV-TWA and TLV-short term exposure limit TLV-STEL for MIBK are 50 and 75 ppm respectively. Butyl Acetate is also widely used its moderate evaporation rate makes it perfect during drying to avoid surface defects of the film blushing cratering Propylene Glycol Mono Methyl Ether Acetate a moderate evaporation solvent.
Compound 9 with an isobutyl group improved IC 50 values to 110 μM 2286 μM and 702 μM for VIM-2 VIM-1 and VIM-5 respectively and also had high ligand efficiency LE values of 054070 for these MBLs. 0mLof waterand transfer to a suitable flask. 65000 μgcapitaday Threshold of Concern.
Silicified Microcrystalline Cellulose Cellulose Acetate. Isobutanol is an alkyl alcohol that is propan-1-ol substituted by a methyl group at position 2. Erythritol 300900 Ethylcellulose 1030 Ethyl Acrylate and Methyl Methacrylate Copolymer Dispersion.
Usually esters are derived from a substitution reaction of carboxylic acid and an alcohol. Methyl isobutyl ketone Hexone 108101 Methyl isocyanate 624839 Methyl methacrylate 80626 Methyl tert butyl ether 1634044 44 -Methylene bis2 -chloroaniline 101144 Methylene chloride Dichloromethane 75092 Methylene diphenyl diisocyanate MDI 101688 44 -Methylenedianiline 101779 Naphthalene 91203. Solvent Other Names Structure.
Autooxidation results in hydroperoxide formation side-chain cleavage and eventually formation of short chain acids such as formic acid all of which could influence the stability of a biopharmaceutical product. Both PEG and PEO have chemical similarities and can be photo-cross-linked by modifying the polymer chain end with acrylates or methacrylates.
This reactivity also includes organic structures within cells and cell nuclei.

Ethylene oxide structure. In this case alkylation and reactions with DNA RNA and proteins occur. The respective half lives were 025 and 2 hours. Ethylene glycol has been synthesized by the oxidation of ethylene with O 2 to ethylene oxide and the subsequent hydration of ethylene oxide to ethylene glycol.
Usually ethylene is supplied from the thermal cracking of naptha from petroleum refining. Nickel single atom and copper nanoparticles used for highly selective tandem electrocatalysis of CO2 to ethylene. Ethylene oxide is used as an intermediate in the production of ethylene glycol and nonionic surfactants.
The tandem electroreduction of CO2 to. Ethylene is the starting material for the preparation of a number of two-carbon compounds including ethanol industrial alcohol ethylene oxide converted to ethylene glycol for antifreeze and polyester fibres and films acetaldehyde converted to acetic acid and. The new research study on Ethylene Oxide market sheds light on the current scope as well as on the upcoming opportunities in the future.
Ethylene oxide is a substance which due to its structure is counted among the very reactive compounds. All these six atoms H-C-H form an angle of 1174 close to the 120 to form a hybridized carbon sp². In English 5035 7988-7997 2011 Non-fouling surfaces produced by gas phase pulsed plasma polymerization of an ultra low molecular weight ethylene oxide containing monomer.
Hydrogel formation can be initiated by either crosslinking PEG by ionizing radiation or by covalent crosslinking of PEG macromers with reactive chain ends. Ethylene glycol is widely used as antifreeze in automobile cooling systems and in the manufacture of human-made fibres low-freezing explosives and brake fluid. The report also focuses on global major leading industry players of Ethylene Oxide market providing information such as.
By Liu Jia Chinese Academy of Sciences. The peak blood ethylene-glycol concentration in ethylene-glycol dosed rats was 11. The peaks at 471 ppm peak d and 364 ppm peak h are assigned to the protons from ethylene glycol units in PEF and methylene oxide units in PTMO segments while those at 721 ppm peak c to the aromatic protons from furan rings respectively.
Propylene glycol also called 12-propanediol resembles ethylene glycol in its physical. Angewandte Chemie International ed. Ethene is a hydrocarbon which has the formula C 2 H 4 or H 2 CCH 2It is a colorless flammable gas with a faint sweet and musky odor when pure.
When crosslinked into networks PEG can have high water content forming hydrogels. ISO 10993-72008 specifies allowable limits for residual ethylene oxide EO and ethylene chlorohydrin ECH in individual EO-sterilized medical devices procedures for the measurement of EO and ECH and methods for determining compliance so that devices may be released. Ethylene glycol is a prominent member of the.
Ethylene oxide found in PEG-4 PEG-7 PEG4-dilaurate and PEG 100 is highly toxiceven in small dosesand was used in World War I nerve gas. Because it is a strained ring ethylene oxide easily participates in a number of addition reactions that result in ring-opening. It is produced commercially from ethylene oxide which is obtained from ethylene.
The molecular structure is based on structures generated from information available in ECHAs databases. Applicability of allowable limits for neonates and infants. Ethylene oxide is an organic compound with the formula C 2 H 4 OIt is a cyclic ether and the simplest epoxide.
Ethylene is widely used in the chemical industry and its worldwide production over 150 million tonnes in 2016 exceeds that of any other organic. And then there is 14-dioxane found in PEG-6 PEG-8 PEG-32 PEG-75 PEG-150 PEG-14M and PEG-20M which on top of being a known carcinogen may also combine with atmospheric oxygen to form explosive peroxidesnot exactly something you want. Cytotoxicity carcinogenicity and mutagenicity of ethylene oxide which have been demonstrated by many in vitro and in vivo tests are.
If generated an InChI string will also be generated and made available for searching. Further the bond is rigid about the C-C bond with high energy process by breaking the π-bond. Ethylene oxide sterilization residuals Amendment 1.
Preparation of Ethylene Glycol C 2 H 6 O 2. A three-membered ring consisting of one oxygen atom and two carbon atomsEthylene oxide is a colorless and flammable gas with a faintly sweet odor. Breathing in relatively high levels of ethylene oxide can cause irritation of the eyes skin and respiratory passages and affect the nervous system for example headaches nausea vomiting memory loss numbness in humans.
A small amount less than 1 is used to control insects in some stored agricultural products and a very small amount is used in hospitals to sterilize medical. Oxidation of the fatty acid moiety while well described in. This information is only displayed if the substance is well-defined its identity is not claimed confidential and there is sufficient information available in ECHAs databases for.
It is the simplest alkene a hydrocarbon with carbon-carbon double bonds. A small amount is used as a fumigant for. As the name suggests it has four atoms of hydrogen bonds that are paired with carbon atoms with a double bond.
The intra-cluster electron transfer towards Pd across the as-formed nanometer metaloxide interface significantly weakens the ethylene adsorption without compromising the. Wu YJ et al. Peak blood ethylene-carbonate and ethylene-glycol concentrations in rats dosed with ethylene-carbonate were 0028 and 23 umolg respectively.
Obermeier B et al. ISO 10993-72008Amd 12019 Biological evaluation of medical devices Part 7. It can be easily synthesized by the anionic ring opening polymerization of ethylene oxide into a range molecular weights and variety of end groups.
Ethylene is a hydrocarbon. For more than 30 years Polymer Engineering Science has been one of the most highly regarded journals in the field serving as a forum for authors of treatises on the cutting edge of polymer science and technology. 87121 Poly ethylene glycol and poly ethylene oxide PEG and PEO are currently FDA licensed and are used for several tissue engineering applications.
Additional background including guidance and a flowchart showing how the standard is applied are also included in. Ethylene oxide is a flammable gas with a somewhat sweet odor. Ethylene glycol and some of its derivatives are mildly toxic.
To understand the structure of global trading the report also gives statistical data on local consumption and global consumption. The polysorbates undergo autooxidation cleavage at the ethylene oxide subunits and hydrolysis of the fatty acid ester bond. It is very reactive with nucleophilic substances such as water alcohols halides amines and sulfhydryl compounds.
Ethylene oxide is a highly flammable gas produced in very large quantities in the United States 53 to 63 billion pounds. Ethylene-glycol was the only ethylene-carbonate metabolite detected. Long-term exposure to ethylene oxide at high levels encountered at some workplaces has also been associated with a small to moderate increase in the incidence of some.
It dissolves easily in waterEthylene oxide is a man-made chemical that is used primarily to make ethylene glycol a chemical used to make antifreeze and polyester. Multifunctional Poly ethylene glycols.
C 3 H 6 O 3. Shop now at NET-A-PORTER.
We are unable to retrieve the vendor information for this entry at this time.

Structure of lactic acid. Lactic acid is an alpha hydroxy acid whose formula is. It consists of an acid functional group -COOH and a hydroxyl group -OH. It has two optical isomers Levo and Dextro making itself a chiral molecule.
Did you know that active people or even a more fidgety person produces more CO2 and Lactic Acid on a daily basis. Structure and Morphology of Polylactic acid Stereocomplex Nano-fiber Shish Kebabs Qing Xie12 Xiaohua Chang12 Qian Qian2 Pengju Pan1 Christopher Y. L--lactic acid Lactic acid 79-33-4.
Lactic acid has a formula of C 3. Rm CH_3CH OHCOOH CH3. You can also browse global suppliersvendorpricesPricemanufacturers of Lactic acid 50-21-5.
Lactic Acid in its most basic form is a build up of chemical compounds in muscle tissue. Lactic acid is a compound formed as a result of energy-producing processes in your body as well as one thats formed on an industrial scale for many applications. When milk sugar lactose undergoes fermentation the product obtained is lactic acid.
The chemical formula of Lactic Acid is rmC_rm3rmH_rm6rmO_rm3rm Its IUPAC name is 2-Hydroxypropanoic acid. Lactic Acid was first discovered in the 1780s by a Swedish chemist named Carl Wilhelm Scheele. This entity has been manually annotated by the ChEBI Team.
C H 3 C H O H C O O H. L-isomers are commonly present among living organisms. This reaction in addition to producing lactic acid also produces nicotinamide adenine dinucleotide NAD that is then used in glycolysis to produce energy source adenosine triphosphate ATP.
Lactic acid is one of the organic acids. These components are not identical because they cannot be superimposed on each other. The lactic acid has a significant part in various biochemical processes.
It is also known as milk acid. Lactic Acid Chemical Formula and Structure. What is Lactic Acid.
Ad Discover the power of beauty. Ad Discover the power of beauty. It has a molecular weight of 90080 gmol and is classified as an alpha-hydroxy acid.
Lactic Acid is an organic acid with chemical formula C 3 H 6 O 3. Lactic Acid DL- is the racemic isomer of lactic acid the biologically active isoform in humans. Lactic acid or lactate is produced during fermentation from pyruvate by lactate dehydrogenase.
An optically active form of lactic acid having S -configuration. At lastLactic acid 50-21-5. Lactic acid 2-hydroxypropanoic acid has the formula C 3 H 6 O 3The molecular structure contains both a terminal carboxylic acid and a hydroxyl group in the 2nd position.
Shop now at NET-A-PORTER. It contains three carbon atoms a hydroxyl group and carboxylic acid as a functional group. The optimal biological activity of lactic acid is.
Asked Jul 6 2020 in Chemistry by Aalaya 476k points icse. Name the type of isomerism exhibited by lactic acid CH3CHOHCOOH giving a reason for your answer. The hydroxyl group is attached to the carbon atom that is adjacent to the carboxyl group.
Structure properties spectra suppliers and links for. This reaction in addition to producing lactic acid also produces nicotinamide adenine dinucleotide NAD that is then used in glycolysis to produce energy source adenosine triphosphate ATP. Lactic Acid L- is the levorotatory isomer of lactic acid the biologically active isoform in humans.
Lactic acid has a carbon atom from which a hydroxyl group -OH and a carboxylic group -COOH is joined along with a methyl group -CH3. He isolated the Lactic Acid present in sour milk. Though it contains hydroxyl functional group it exhibits more acidic properties.
Visit ChemicalBook To find more Lactic acid 50-21-5 information like chemical propertiesStructuremelting pointboiling pointdensitymolecular formulamolecular weight physical propertiestoxicity informationcustoms codes. Lactic acid 2-Hydroxypropanoic acid is an example of a compound which shows optical isomerism It contains one asymmetric carbon atom. One is the mirror image of the other.
The orientation of the hydroxyl group allows for two optical isomers l- or S lactic acid and d- or R lactic acidChemical routes typically produce racemic lactic acid while biological routes commonly approach. Two 3 dimensional structures are possible for Lactic acid. It is found in cottage cheese leban sour milk yogurt and Koumiss.
The IUPAC name of lactic acid is 2-hydroxypropanoic acid. Lactic acid or lactate is produced during fermentation from pyruvate by lactate dehydrogenase. Li2 1 State Key Laboratory of Chemical Engineering College of Chemical and Biological Engineering Zhejiang University 38 Zheda Road Hangzhou 310027 China 2 Department of Materials Science and Engineering Drexel University.
The chemical formula of the lactic acid is C3H6O3. The structure of lactic acid is shown in Fig. It contains an asymmetric carbon atom due to which lactic acid exists in two forms as L lactic acid and D-lactic acid.
Chemical Structure of Lactic Acid.
Propan-2-ol can be dehydrated to give propene by heating it with an excess of concentrated sulphuric acid at about 170C. The acids arent written into the equation because they serve as catalysts.

File Propan 2 Ol Alt Png Wikimedia Commons
The tertiary structure only Question 5 A single 10 g piece of iron metal is completely submerged in 50 mL of 02 M nitric acid HNO 3 contained in a 100 mL beaker.
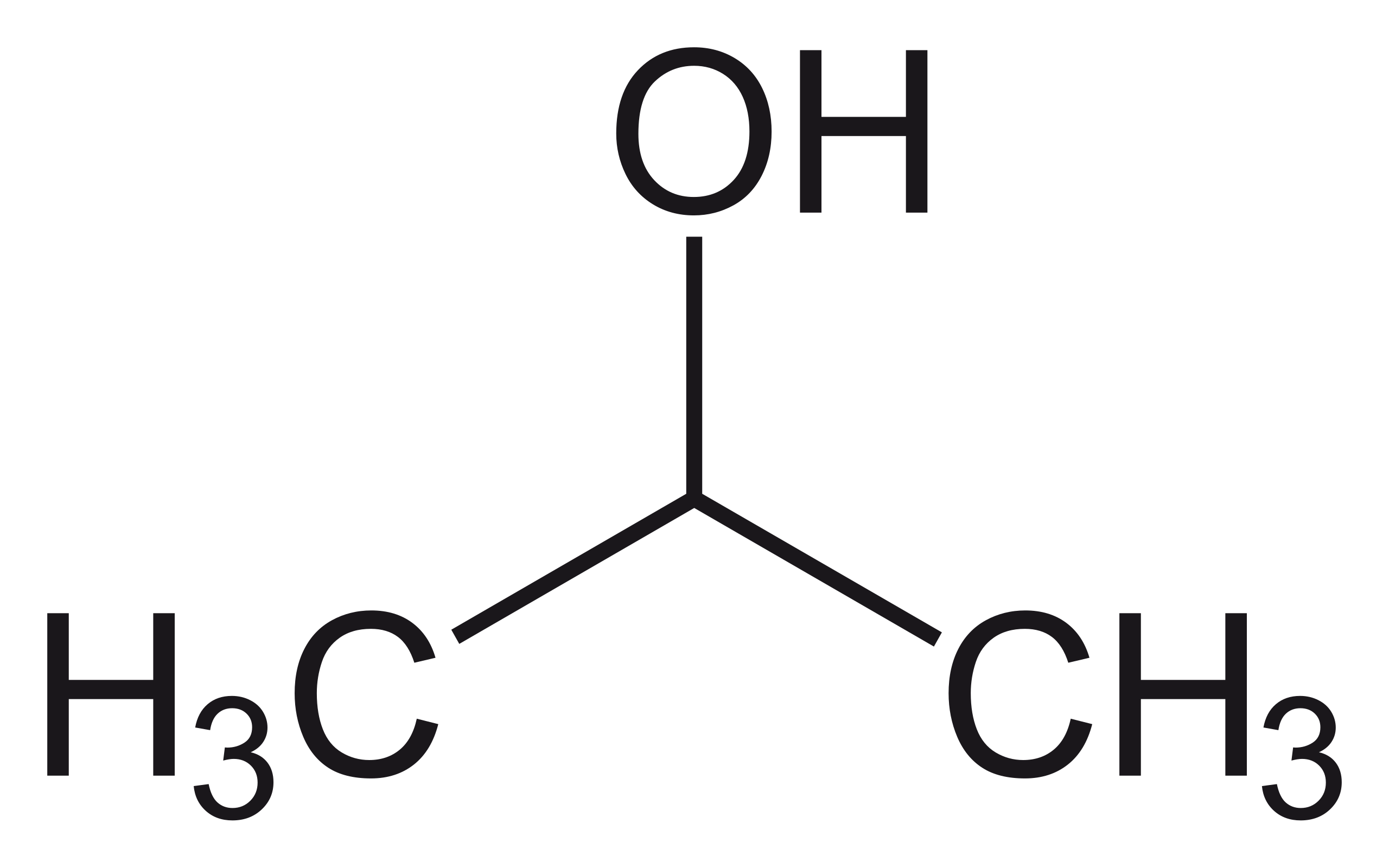
Structure of propan-2-ol. I CH 3 CH 2 CH 2 CH 2 OH ii 2-butanol iii 2-methyl-l-propanol Delhi 2012 Answer. The two propanol isomers consist of propan-1-ol and propan-2-ol also known as isopropyl alcohol which are distinguished by the placement of an oxygen atom either on the terminal carbon atom or the central carbon atom respectively. Active site structure Aeropyrum pernix.
Miscible with water alcohol ether chloroform Alfa Aesar 36644. So the condensed structural formula of propan-2-ol is CH_3CHOH. It has a role as a plant metabolite.
Elimination reactions in halogenoalkanes and alcohols when a displayed formula is required when a skeletal structure is. Table 1 Concentration of. FA9 can be propan-1-ol or propan-2-ol depending on whether the iodoform test test3 gives the yellow ppt of CHI3.
Disinfectant arbitrary units. The experiment is performed at standard laboratory conditions SLC. The dehydration of propan-2-ol.
DT-A61 A65 A68 A22 OUCMDZ-3118 and bingchenggensis ULS14 were reported in a roll during 2018 and 2019 Figure 2. Does this mean adding I2 in excess. A Illustrate the following name reactions.
When mixed with a more polar solvent such as 2-propanol propan-2-ol the mixture is sufficiently polar to carry the ibuprofen through the inner layer of the skin but not so polar that it will not dissolve ibuprofen. The use of propan-2-ol isopropanol isopropyl alcohol should be allowed for manufacturing the additives curcumin E 100 and paprika extract E 160 c in line with JECFA specifications as this particular use has been considered safe by the Authority 10. 1374-138 Food and Agriculture Organization of the United Nations Propan-2-ol.
Interpret evidence about chemical equilibrium systems oxidation and reduction properties and structure of organic materials and chemical synthesis and design to draw conclusions based on analysis. A total of 18 new indolocarbazole alkaloids 11 28 isolated from Streptomyces sp. Assume that an excess of oxidising agent is used.
Table 1 shows the students results. What is the IUPAC name for isopropyl alcohol. For propan-2-ol CH 3 CHOHCH 3 In most cases the use of sticks to represent C H bonds in a structure should not be penalised.
This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. A Lewis Structure electron dot diagram shows us how the bonding and non-bonding valence electrons are arranged around the atoms. What might FA10 and FA11 be.
This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. The primary structure only D. Draw the structure and name the product formed if the following alcohols are oxidized.
Take the test now. And structure of organic materials and chemical synthesis and design to identify trends patterns relationships limitations or uncertainty 4. In some molecules IUPAC names consider the positions where functional groups are located in the molecule.
1 - Phenyl - 2 - propanol. At 254nm 013 AU max. If you like you could write for example conc H 2 SO 4 over the top of the arrow.
IUPAC name Propan-2-ol. The three disinfectants used by the student were Lysol propan-2-ol and ammonia. Ibuprofen is synthesized from 2-methylpropylbenzene which can be made from compounds.
The use of ethanol in replacement of propan-2-ol in the manufacturing of gellan gum E 418 should be permitted where the final product. As an isopropyl group linked to a hydroxyl group it is the simplest example of a secondary alcohol where the alcohol carbon atom is attached to two other carbon atoms. The reaction that occurs is Fes 2HNO 3aq FeNO 3 2aq H 2g The rate of the reaction would most likely increase.
Some chemical compounds do not have common namesSome. A condensed structure uses parentheses to show that polyatomic groups within a formula are attached to the nearest non-hydrogen atom on the left. Identify any branches or side-chains in the condensed structural formula or.
The IUPAC name is directly related to its chemical structure. The exceptions to this when sticks will be penalised include structures in mechanisms where the C H bond is essential eg. In other words IUPAC names consider the functional groups side chains and other special bonding patterns in the molecule Examples.
The chemical structure C 3 H 8 O exists as several isomers of propanol as well as the isomer methoxyethane. Concentrated phosphoricV acid H 3 PO 4 can be used instead. Iii Propene to propan-2-ol.
4-06-00-03192 Beilstein Handbook. B Give a. It has a role as a plant metabolite.
Marking guide and solution. I Reimer-Tiemann Reaction ii Williamson Synthesis. It is a structural isomer of 1-propanol and.
Structureactivity relationship analysis of compounds 9 10 and seven known analogs showed C-6 chlorine as an essential pharmacophore in their cytotoxic activities. Propylene on esterification in the presence of sulphuric acid undergoes. Isopropyl alcohol IUPAC name propan-2-ol and also called isopropanol or 2-propanol is a colorless flammable chemical compound chemical formula CH 3 CHOHCH 3 with a strong odor.
Do you understand this. 2 The liquid culture the student transferred. 13776 Kaye Laby No longer updated Experimental Solubility.
At 230nm 025 AU max. Isopropyl Alcohol Structure C 3 H 8 O. Notice that the position of the -OH group is determined by where the HSO 4 group was attached.
Alpha-terpineol is a terpineol that is propan-2-ol substituted by a 4-methylcyclohex-3-en-1-yl group at position 2. A primary alcohol NAD an aldehyde NADH H catalytic mechanism involves a proton relay modulated by the coupled ionization of the active site Lys155Tyr151 pair and a NAD ribose 2-OH switch other active site residues are Ser138 and Trp144 ionization properties substrate binding overview Scaptodrosophila lebanonensis. But the condensed structure of ethanol is CH_3CH_2OH while for dimethyl ether the condensed structure is CH_3OCH_3.
Methoxyethane is also an isomer of C. Number of colonies of bacteria. Isopropyl Alcohol Synthesis- C 3 H 8 O.
You get propan-2-ol rather than propan-1-ol because of the way the sulphuric acid originally added across the double bond in propene. 0785 gmL Alfa Aesar L10181 36644 19397 22906 40983 39194 41840. Isopropyl Alcohol Structure C3H8O.
Draw the 2-dimensional full display structural formula for propan-2-ol 2-propanol which has the condensed structural formula or semi-structural formula CH 3 2 CHOH Step 1. Why is it so important to make sure you decant all of the isopropanol supernatant before. Molecular Weight Molar Mass.
CH 3 CHOHCH 3. More complicated alkyl hydrogensulphates react with water in exactly the same way. At 220nm 100 AU max.
Could someone please explain all reactions happening in the tests especially for FA11. For test 3 I am not sure why they want us to add I2 until the solution turns orangered. C 3 H 8 O.
