The dipole is shown by an arrow with a cross at one end. The hydrogen chloride molecule has a dipole two poles which consists of a pair of opposite charges separated from each other.
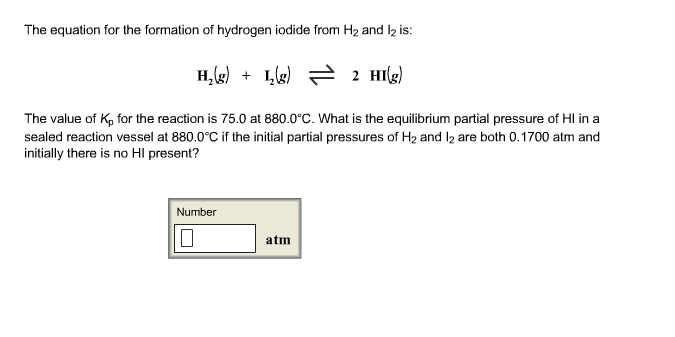
Solved The Equation For The Formation Of Hydrogen Iodide Chegg Com
Meanwhile the thyrocyte endoplasmic reticulum synthesizes two key proteins TPO and Tg.
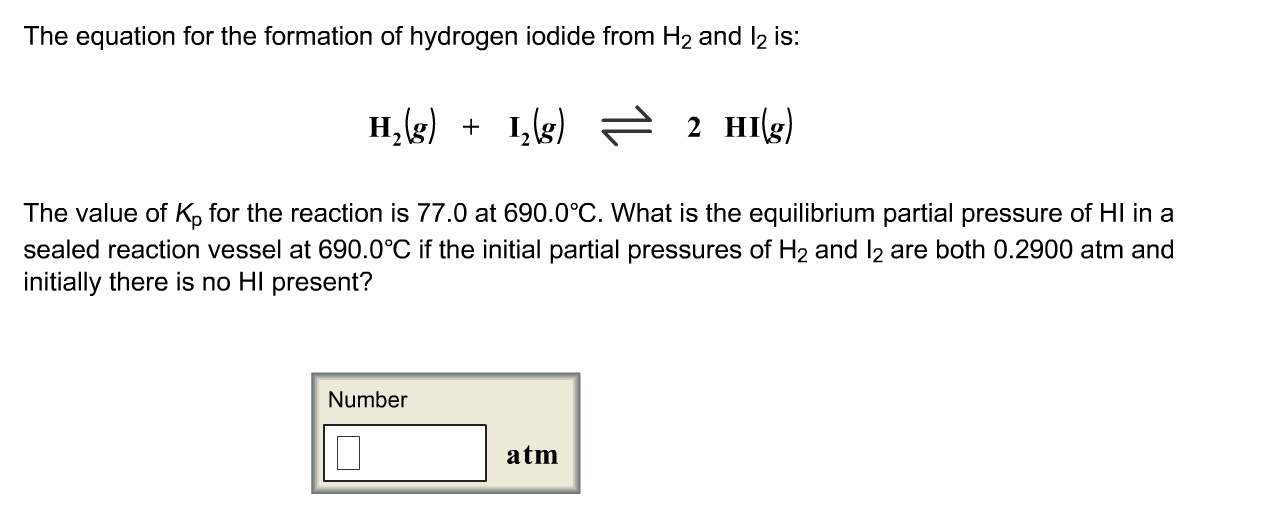
Formula of hydrogen iodide yields hydrogen iodide. Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction. It is present in teeth and bone and helps in metabolic processes. A metal plus a polyatomic ion yields an ionic compound.
For purposes of computing a formula mass it is helpful to rewrite the formula in the simpler format Al 2 S 3 O 12. Single or multiple bonds between carbon atoms are nonpolar. VIA O O2 oxide ion I I iodide ion S S2.
Formaldehyde and acetic acid have the same empirical formula CH 2 O. Silver iodide should be stored in cool dark areas away from the above materials. We will learn something about kinetics by experimentally determining a and b and by estimating k as a pseudo.
The cross is near the end of the molecule that is partially positive and the arrowhead is near the partially negative end of the molecule. The hydrogen peroxide produced subsequently oxidizes colorless iodide ion to yield brown iodine which may be visually detected. Before shipping silver iodide consult with the regulatory requirements of the US Department of Transportation.
Propene yields two products however only one predominates as. 1302g of C 8 H 17 OH 1-octanol formula mass 1302 amu 4549g of HgI 2 mercuryII iodide formula mass 4599 amu 320g of CH 3 OH methanol formula mass 320 amu and 2565g of S 8 sulfur formula mass. Explain the fact that in alkyl aryl ethers alkoxy group.
Salt with potassium iodide. Tg is a 660kDa glycoprotein secreted into the lumen of follicles whose tyrosyls serve as substrate for iodination and hormone formation. Thus two cysteine molecules combine into a less reactive molecule of cystine.
Molecular formulae indicate the simple numbers of each type of atom in a. Phosphoric Acid is an acid-containing four atoms of oxygen one atom of phosphorus and three atoms of hydrogen. D From alkenes i Addition of hydrogen halides.
It is also known as phosphoricV acid or orthophosphoric acid. To Learn about the structure of Acetic acid its preparations chemical physical properties uses and FAQs. The low reactivity of the disulfide bond protects skin from destruction by air water and in fact all the commonly occurring.
Clock-wise from the upper left. Ii directs the incoming substituents towards ortho and para positions in. TPO sits at the apical plasma membrane where it reduces H2O2 elevating the oxidation.
Potassium iodide given 12 and 24 hours before exposure yields a 90 and 70 protectant effect respectively. In its liquid form it appears as a clear colourless solution and in its solid form it. Write the equation of the reaction of hydrogen iodide with i1-propoxypropane iimethoxybenzene and iiibenzyl ethyl ether Ans.
However potassium iodide administered 1 and 3 hours after exposure results in an 85 and 50 protectant effect respectively. The first equation depicts the oxidation of glucose in the urine to yield glucolactone and hydrogen peroxide. The iodide reacts with magnesium to produce a Grignard product η 5-cpFeη 5-cp-MgI which reacts in a coupling reaction to yield diferrocene.
This product can also be produced by the reaction of iodine with the mercury compound shown earlier which yields the iodide. This is the actual chemical formula for formaldehyde but acetic acid has double the number of atoms. I activates the benzene ring towards electrophilic substitution.
H 3 O hydronium. Hydrogen iodide H I is a diatomic molecule and hydrogen halide. It allows the chemist to determine the amount of product that will form from a given amount of reactants or the amount of one reactant that is needed to react completely with some specific amount of the other reactant.
For small dry spills of silver iodide collect the material and deposit in sealed. Following the approach outlined above the formula mass for this compound is calculated as follows. Where k is the rate coefficient left I- right is the iodide concentration left H_2 O_2right is the hydrogen peroxide concentration and a and b are the order of the reaction with respect to iodide and hydrogen peroxide respectively.
Iodine can combine with sulfhydryl the sulfur-hydrogen bond of cysteine removing the hydrogen and by means of this oxidation setting the stage for sulfur atoms to bind to each other. An alkene is converted to corresponding alkyl halide by reaction with hydrogen chloride hydrogen bromide or hydrogen iodide. The formula for this compound indicates it contains Al 3 and SO 4 2 ions combined in a 23 ratio.
Acetic Acid CH3COOH- Acetic Acid is an organic compound with formula CH3COOHVinegar is a water solution of acetic acid containing 5-8 of acetic acid by volume. Although an enormous number of reactions can be carried out to produce. Potassium iodide administered more than 6 hours after exposure is thought to have a negligible protectant effect.
Some strips include an additional substance that reacts with iodine to produce a more distinct color change. An acid-base reaction is one in which a hydrogen ion H is transferred from one chemical species to anotherSuch reactions are of central importance to numerous natural and technological processes ranging from the chemical transformations that take place within cells and the lakes and oceans to the industrial-scale production of fertilizers pharmaceuticals and other. Go ahead and enjoy some dirty incest kinky fun with desi aunty sex.
It has a pungent smell and a sour taste. C 2 H 3 O 2-acetate OAc- C 2 O 4 2-oxalate. Formulas and Names of Some Polyatomic Ions NH 4 ammonium CO 3 2 carbonate H 3O hydronium HCO 3 hydrogen carbonate bicarbonate OH hydroxide OCN cyanate CN cyanide SCN thiocyanate O 2 2-2peroxide S 2O 3 thiosulfate N 3-azide CrO 4 2 chromate NO 2.
Formulas and Names of Some Polyatomic Ions. Phosphoric Acid is a weak acid with chemical formula H 3 PO 4. Visit BYJUS for more content.
Each sample contains 602 10 23 molecules or formula units100 mol of the compound or element. A metal plus a polyatomic ion yields an ionic compound. Intracellular iodide is then transported in the lumen of thyroid follicles.
Silver iodide may form explosive compounds with sodium potassium acetylene ammonia and hydrogen peroxide. Likewise the empirical formula for hydrogen peroxide H 2 O 2 is simply HO expressing the 11 ratio of component elements. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid a strong acidHydrogen iodide and hydroiodic acid are however different in that the former is a gas under standard conditions whereas the.