Test cycles according to this standard usually run 168 hours. The first number under the column headed RQ is the reportable quantity in pounds.
Lead Chromate Precipitate 2 Of 3 Stock Image C036 3126 Science Photo Library
SnOH2 tin II hydroxide 28.
Lead 2 chromate. Similar calculation for the leadII nitrate yields. When potassium chromate is reacted with silver nitrate an insoluble white precipitate of silver chromate is formed along with potassium nitrate. Lead paint or lead-based paint is paint containing lead.
Lead reacts with air in the presence of moist and carbon dioxide forming a passivating layer. 2 lead IV sulfite 2. HPO 4 2.
AlClO33 aluminum chlorate 21. Lead sulfate PbSO 4 also known as anglesite is used in a paint pigment known as sublimed white lead. 2 If an employee is exposed to lead for more than 8 hr in any work day the permissible exposure limit as a time weighted average TWA for that day shall be reduced according to the following formula.
Lead acetate is a lead compound which has a sweet taste and is called sugar of lead An electron is a negatively charged particle that orbits the positively charged nucleus of the. SrNO32 strontium nitrate 27. IUPAC Name older name Formula.
An increase in the incidence of lung cancer has been observed among workers in industries that produce chromate and manufacture pigments containing chromate. The chemical equation for this reaction is given by. Lithium carbonate Li 2 CO 3 2510 2.
Both malleable and ductile though with little tenacity. LeadII sulfide PbS 310 28. The oxide is soluble in excess.
The uptake of hexavalent chromium compounds through the airways and digestive tract. Q 00000938 M Pb 2000050 M CrO 4 2- 469 x 10-8 Q is greater than K sp so a precipitate of leadII chromate will form. Bicarbonate HCO 3.
Magnesium ammonium phosphate MgNH 4 PO 4 25. LeadII sulfate PbSO 4 1610 8. Carcinogenic lead chromate induces DNA double-strand breaks in human lung cells.
PbSO42 lead IV sulfate 19. LeadII fluoride PbF 2 2710 8. Maximum permissible limit in ugcu m400 divided by the number of hours worked in the day.
C 2 for PbNO 3 2 00000938 M. Type 2 Chem Film Chromate Conversion Coating Tank System. 3 When respirators are used to supplement engineering and work practice controls to comply with.
Department of Defense was the first to use TCP-HF trivalent chromium hex-free and this trivalent chromate conversion coating has become such an accepted standard that a detailed specification MIL-DTL-5541 included it. Ionic bonds also melt at high temperatures. It is one of the main.
The number in parentheses is the metric equivalent in kilograms. SnCN2 tin II cyanide 18. It is easily fusible forms alloys with other metals and is an ingredient of solder and type metal.
Chromium is the proximate clastogenic species for lead chromate-induced clastogenicity in human bronchial cells. There are regulations in place banning the use of lead paint. Write the chemical formula for the following ionic compounds.
In Europe regulators are trying. Lead is added to paint to accelerate drying increase durability maintain a fresh appearance and resist moisture that causes corrosion. PbII reacts with hydroxide forming leadII oxide.
Advanced Plating Technologies no longer offers chromate services as a final finish. CuClO42 copper II chlorate 25. Testing per MIL-DTL-5541 specification.
Search by reactants AgNO 3 K 2CrO 4. Learn more about Quia. CH 3 COO C 2 H 3 O 2.
Ionic bonds are atomic bonds created by the attraction of two differently charged ionsThe bond is typically between a metal and a non-metal. Lithium phosphate Li 3 PO 4 3210 9. The chromate ion is the predominant species in alkaline solutions but dichromate can become the predominant ion in acidic solutions.
Jefferson Lab US. Very few countries have completely banned all uses of lead paint and even in the US Canada and Europe it is legal to use industrial lead paints for many applications. IO 2 4 lead IV hydrogen chromate PbHCrO 4 4 lithium peroxide Li 2O 2 cobalt II perchlorate CoC ℓO 4 2 arsenic V thiosulfate As 2S 2O 3 5 gold III fluoride AuF 3 calcium permanganate CaMnO 4 2 sodium peroxide Na 2O 2 aluminum thiocyanate Aℓ SCN 3 strontium cyanate SrOCN 2 copper.
Li 2 CO 3. Uncountable A heavy pliable inelastic metal element having a bright bluish color but easily tarnished. ChromiumVI compounds such as calcium chromate zinc chromates strontium chromate and lead chromates are highly toxic and carcinogenic in nature.
Mg3PO42 magnesium phosphate 20. The structure of the bond is rigid strong and often crystalline and solid. SnCO32 tin IV carbonate 24.
2 Pb s O 2 g 2 PbO s Reaction of lead with bases. This activity was created by a Quia Web subscriber. LeadII iodide PbI 2 7110 9.
When heated in a stream of air lead burns 8. Lead chromate PbCrO 4 also known as crocoite is used to produce chrome yellow paint. Lead silicate PbSiO 3 is used to make some types of glass and in the production of rubber and paints.
These molecules are called lead compounds or sometimes lead salts. LeadII chromate PbCrO 4 2810 13. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A.
LeadII hydroxide PbOH 2 1210 15. Xie H Wise SS Holmes AL Xu B Wakeman T Pelsue SC Singh NP Wise JP. BaOH2 barium hydroxide 22.
What is the product of AgNO3 K2CrO4. Please use the below content for reference only Please use the below content for reference only Aluminum chromate conversion coatings often referred to as chemical film or under the trade names Alodine or Irridite produce a thin coating in the range of 000001-000004 inches in thickness. Lead oxide lead chromate and lead acetate are all molecules formed when lead atoms combine with atoms of other elements to form molecules.
NH42CrO4 ammonium chromate 17. As pigment leadII chromate Pb Cr O 4 chrome yellow LeadIIIV oxide Pb 3 O 4 red lead and leadII carbonate Pb C O 3 white lead are the most common forms. Cr 2 O 7 2.
Chromium III on the other hand is an essential nutritional supplement for animals and humans and has an important role in glucose metabolism. PMC free article Google Scholar 160. Lithium fluoride LiF 3810 3.
The predominance diagram shows that the position of the equilibrium depends on both pH and the analytical concentration of chromium. Lead nitrate PbNO 3 2 is used to make fireworks and other pyrotechnics. 2 AgNO3aq K2CrO4aq Ag2CrO4s 2 KNO3aq.
PbC 2 O 4. Pickerington High School Central. Atomic number 82 symbol Pb from Latin plumbum countable nautical A plummet or.
Create your own activities. In aqueous solution chromate and dichromate anions exist in a chemical equilibrium. Chromate CrO4 2-cyanide CN-dichromate Cr2O7 2-hydrogen carbonate bicarbonate HCO3-hydrogen sulfate bisulfate HSO4-hydrogen phosphate biphosphate HPO4 2-hydroxide OH-hypochlorite ClO-iodate IO3-nitrate NO3-nitrite NO2-oxalate C2O4 2-perchlorate ClO4-periodate IO4-permanganate MnO4-peroxide O2 2-phosphate PO4 3-phosphite PO3 3-silicate SiO4 4-sulfate SO4 2-sulfite SO3 2-thiocyanate SCN.
Lead dissolves slowly in cold alkalis to form plumbites. FeHSO32 iron II bisulfite 26. HgCrO4 mercury II chromate 23.
A few countries including the Philippines have regulated the lead content of both residential and industrial paints. The layer is most likely lead hydroxy carbonate 8. CrO 4 2.
Occupational exposure to lead is one of the most prevalent overexposures. 2 CrO 2 4 2 H Cr 2 O 2 7 H 2 O. Industries with high potential.
Using the initial concentrations calculate the reaction quotient Q and compare to the value of the equilibrium constant K sp. Calcium chromate chromium trioxide lead chromate strontium chromate and zinc chromate are known human carcinogens.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Give either the name or formula with the correct charge for each of the cations.
Potassium Chromate Crk2o4 Chemspider
Parentheses and a subscript are not used unless more than one of a polyatomic ion is present in the formula unit eg calcium sulfate CaSO 4 not CaSO 4.
Formula potassium chromate. Write the Formula Formula Unit for the following Compounds. Sodium nitrite NaNO2 31. The salt is popular in the laboratory because it is not.
This way students can see that the ions combine in whole number ratios in order to produce a neutral chemical species. Sodium thiosulfate ____Na2S2O3_____ 3. Use the stock form for the transition metals.
BaClO42 barium perchlorate 11. ZnHCO32 zinc bicarbonate 15. Identify the charges Mg.
Cr 2 K 2 0 7 the hexavalent form of. If the subscript is a 1 it does not need to be written. Its important to remember that common names are inaccurate and vary from one place and time to another.
This is described above if you have forgotten. Potassium Chromate is a yellowish crystalline inorganic compound that emits toxic chromium fumes upon heating. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.
Many potassium salts are of utmost importance including the hydroxide nitrate carbonate chloride chlorate. FeNO22 iron II nitrite 9. The chemical formula calculator also contains the names of a range of.
S 2 O 3 2-Carbonate. KNO3 potassium nitrate 8. Chemical Formula Nomenclature Practice.
Sodium sulfate Na 2 SO 4. Potassium chromate is highly corrosive and is a strong oxidizing agent. KHCO3 potassium bicarbonate 7.
Copy this to my account. O 2 2--3 ions. In its solid form potassium chloride.
C 2 O 4 2-Peroxide. 31 cobalt III chromate Co2CrO43 32 ammonium oxide NH42O 33 potassium hydroxide KOH 34 lead IV sulfate PbSO42 35 silver cyanide AgCN 36 vanadium V nitride V3N5 37 strontium acetate SrC2H3O22 38 molybdenum sulfate MoSO43 39 platinum II. SnCN2 tin II cyanide.
Iron II chloride 18. At work request a material safety data sheet to help identify alternatives that are safe hence avoiding contact with material containing chromates. Formula writing rules to write the correct chemical formulas for each compound.
AgNO3 silver nitrate 10. You may remember that that is done by adding acid. Potassium is an essential constituent for plant growth and is found in most soils.
An alloy of sodium and potassium NaK is used as a heat-transfer medium. Chromate containing minerals are rare. When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions.
Ionic Compounds Summary Name the following compounds. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Avoid all of these.
MgOH2 magnesium hydroxide 9. Each card has the name in both the old system and the stock system. Rare potassium chromate minerals and related compounds are found in the Atacama desert.
The solution turns yellow as potassium chromateVI is formed. In the event such products are used goggles should be worn at all times. It is an ionic compound with two potassium ions K and the negatively charged dichromate ion Cr2O7- in which two hexavalent chromium atoms with oxidation state 6 are each attached to three oxygen atoms as well as a bridging oxygen atom.
Sulfur dioxide ____SO2_____ 2. Ionic Compounds Naming and Formula Writing. LiCIO3 lithium chlorate 10.
Potassium chloride is an ionic salt featuring a bond between an alkali metal and a halogen. B the chemical formula of the compound appears after the arrow. Chromate CrO 4 2-cyanide CN-dichromate Cr 2 O 7 2-dihydrogen phosphate H 2 PO 4 - or H 2 O 4 P-formate CHO 2-or HCOO-or CHOO-hydrogen sulfate or bisulfate HSO 4-hydrogen sulfite or bisulfite HSO 3-hydrogen phosphate HPO 4 2-hydroxide OH-hypochlorite ClO-nitrate NO 3-nitrite NO 2-oxalate C 2 O 4 2-perchlorate ClO 4-permanganate MnO 4-peroxide O.
This substance is used in the manufacture of dyes and in textile dyeing processes. All that is left is to convert the yellow potassium chromateVI solution into orange potassium dichromateVI solution. NO 2 barium.
CdOH2 cadmium hydroxide 13. Na2CrO4 sodium chromate 19. PbCO32 lead II carbonate 12.
E-mail to a friend. Give the formula for the following. 2 reduce it to.
You should complete this by Sunday. Crocoite PbCrO 4 which can occur as spectacular long red crystals is the most commonly found chromate mineral. Even so you rarely ask someone to pass the sodium chloride at the dinner table.
Give the formula for each compound. H 3 O ferric. Notes about the Materials The list of ions was based on a dry lab from JA.
Potassium dichromate K 2 Cr 2 O 7 is a common inorganic chemical reagent most commonly used as an oxidizing agent in various laboratory and industrial applications. Determining the formula for Magnesium Fluoride. Complete these in lab and on your own time for practice.
Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound. Cr 2 O 7 2-Thiosulfate. Potassium carbonate 2K CO 3 2- K 2 CO 3.
K2Cr2O7 potassium dichromate 6. Sodium chloride and potassium chloride. SnSO4 tin II sulfate 14.
If there is a common subscript such as 2 as in. It is a crystalline ionic solid with a very bright red-orange color. Chemical or scientific names are used to give an accurate description of a substances composition.
It is denoted by the chemical formula KCl and is made up of potassium cations and chloride anions in a 11 ratio. Cu 2CO3 copper I carbonate 8. NiS nickel II sulfide Write the chemical formula for each of the following compounds.
Compound Name Type of Compound. Hg2NO22 mercury I nitrite 16. Beran - Laboratory Manual for Principles of General Chemistry.
Potassium chromate primarily affects the nose throat and lungs causing. This reaction is also described further up the page. Among them is lópezite.
Potassium carbonate K 2 CO 3 2. NH42CrO4 ammonium chromate 17. The chemical formula for potassium dichromate is K 2 Cr 2 O 7 and the molar mass is calculated to be 294185 gmol.
Write Formula Unit For the Below. As with all hexavalent chromium compounds it is acutely and chronically harmful to health. Which is also.
Cross the Charges Mg. NH42SO4 ammonium sulfate 20. Unfortunately there is a problem here.
Potassium chloride is characterized by a colourless crystalline appearance and an odourless smell.
Lead absorption is influenced by factors such as age and physiological status. Lead Chromate is a yellow orange or red colored crystalline inorganic compound that emits toxic chromium fumes upon heating.

Pdf Precipitation Of Lead Ii Chromate Folk Narongrit Academia Edu
The less water soluble the chromate the higher was its elimination via the feces.

Lead chromate in water. It is expected that chromate use in open recirculating cooling systems will be banned altogether by the end of 1993. Uncountable A heavy pliable inelastic metal element having a bright bluish color but easily tarnished. In the human body the greatest.
First determine the overall and the net-ionic equations for the reaction that. Pb2aq 2NO3-aq 2Kaq 2I-aq. LeadII is precipitated by chromate under acidic conditions.
Answer 1 of 4. 815 x 10-4. The membrane has a tight pore structure that effectively removes up to 99 of all contaminants and impurities such as total dissolved solids chemicals.
When used as oxidizing agents or titrants in a redox. Lead chromate primarily affects the lungs causing shortness of. It is easily fusible forms alloys with other metals and is an ingredient of solder and type metal.
ChromiumVI compounds such as calcium chromate zinc chromates strontium chromate and lead chromates are highly toxic and carcinogenic in nature. Lead reacts vigorously with fluorine F 2 at room temperature and chlorine Cl 2 when heated forming the corresponding leadII halides 8. Both malleable and ductile though with little tenacity.
As an Anti-knock Agent Tetraethyl lead is utilized as an anti-knock. 271 synonyms for lead. Li 2 CO 3.
This substance is used in printing inks paints and to color vinyl rubber and paper. Atomic number 82 symbol Pb from Latin plumbum countable nautical A plummet or. Class 1A chem film is thicker and darker than the lighter thinner Class 3 chem film.
The reaction is as follows. Chromate and dichromate salts of heavy metals lanthanides and alkaline earth metals are only very slightly soluble in water and are thus used as pigments. Guide to watercolor pigments.
Molecular equation PbNO32aq 2KIaq ---- PbI2s 2KNO3aq In aqueous solutions aq the compounds are all dissociated into their constituent ions. Chromate salts contain the chromate anion. In Europe regulators are trying to ban paint ingredients containing lead on a chemical-by-chemical basis and have banned the use of lead chromate pigments.
Synonyms for lead in Free Thesaurus. This insoluble solid is the precipitate and it has a red-brown color. This characteristic provides.
Lead is added to paint to accelerate drying increase durability maintain a fresh appearance and resist moisture that causes corrosion. Aluminum chromate conversion coatings often referred to as chemical film or under the trade names Alodine or Irridite produce a thin coating in the range of 000001-000004 inches in thickness. Calcium chromate solubility is 170 gL.
When inhaled hexavalent chrome is a suspected carcinogen. Therefore as of May 1990 the use of chromate in comfort cooling towers was banned by the EPA. Lead paint or lead-based paint is paint containing leadAs pigment leadII chromate Pb Cr O 4 chrome yellow LeadIIIV oxide Pb 3 O 4 red lead and leadII carbonate Pb C O 3 white lead are the most common forms.
The legal limit for a contaminant reflects the level that protects human health and that water systems can achieve using the best available technology. When silver nitrate and potassium chromate solutions are mixed together then there is formation of silver chromate and potassium nitrate. When one takes up large amounts of calcium.
Chromium III on the other hand is an essential nutritional supplement for animals and humans and has an important role in glucose metabolism. What color is the precipitate between silver nitrate and potassium chromate. Pb s F 2 g PbF 2 s Pb s Cl 2 g PbCl 2 s LeadII is precipitated by chloride bromide and.
Please place orders before the 5th of December to ensure delivery. The class of the chemical film will also have an effect on the chromate finish. Aluminum chromate conversion coatings are amorphous in structure with a gel-like composition hydrated with water.
The Complete ionic equation shows all the ions present in solution separately. PbC 2 O 4. Pb 2 aq CrO 4 2 aq PbCrO 4 s yellow Reaction of lead with halogens.
This prevents lead from dissolving in drinking water and thereby prevents it from entering the human body. Efforts to restrict the use of lead paint date back to the 1920s but it was not banned for residential use in the US. Exposure to lead occurs mainly via inhalation of lead-contaminated dust particles or aerosols and ingestion of lead-contaminated food water and paints 173 174.
Lead chromate is highly corrosive and is a strong oxidizing agent. The uptake of hexavalent chromium compounds through the airways and digestive tract. As the silver nitrate solution is slowly added a precipitate of silver chloride.
Improper acid activation or pH levels in tanks can. EPA sets legal limits on over 90 contaminants in drinking water. Absorbed chromium was retained in the spleen and bone marrow in all three cases and also in the liver and.
Will a precipitate of leadII chromate form. The lead-containing pigment chrome yellow was used for a very long time before environmental regulations discouraged its use. Calcium carbonate has a positive effect on lead water pipes because it forms a protective leadIIcarbonate coating.
Adults absorb 35 to 50 of lead through drinking water and the absorption rate for children may be greater than 50. 51Chromium labelled sodium zinc and lead chromates were studied. Reverse Osmosis commonly abbreviated to RO is a water treatment method traditionally known for removing salt from seawater it is also used to purify drinking water by forcing untreated water molecules through a semipermeable membrane.
EPA rules also set water-testing. As Soldering Material Due to its low melting temperature and wide availability lead along with tin and other alloys act as the most commonly used solder material for electronics. Watertest Systems will be closed from December 23rd until January 4th.
Calcium phosphate solubility is 20 mgL and that of calcium fluoride is 16 mgL. What are synonyms for lead. Ineffective rinses can lead to contaminated tanks.
The preparation stages of chromate conversion coatings are important too. 250 mL of 00020 M potassium chromate are mixed with 750 mL of 0000125 M leadII nitrate. Go in front of head be in front be at the head of walk in front of guide conduct steer escort precede usher pilot.
The colored linksat the top of the screen take you to detailed information on modern watercolor pigments based on evaluations of over 750 commercial watercolor paints the most comprehensive watercolor paint information available on the InternetIf you dont see the color links click here The information on this site is built around a simple strategyfor. Table 1173 - Reportable Quantities of Hazardous Substances Designated Pursuant to Section 311 of the Clean Water Act. Sodium chromate and the less soluble zinc chromate were absorbed into the blood resulting in increased urinary excretion of chromium.
K sp of leadII chromate is 18 x 10-14. The most recent concern relating to chromate treatment involves chromate present in cooling tower drift. White lead lead sulfate and lead chromate are used as coloring elements in paints and ceramic glazes notably in the colors red and yellow.
Give the formula for the following. E-mail to a friend.
Formula writing rules to write the correct chemical formulas for each compound.
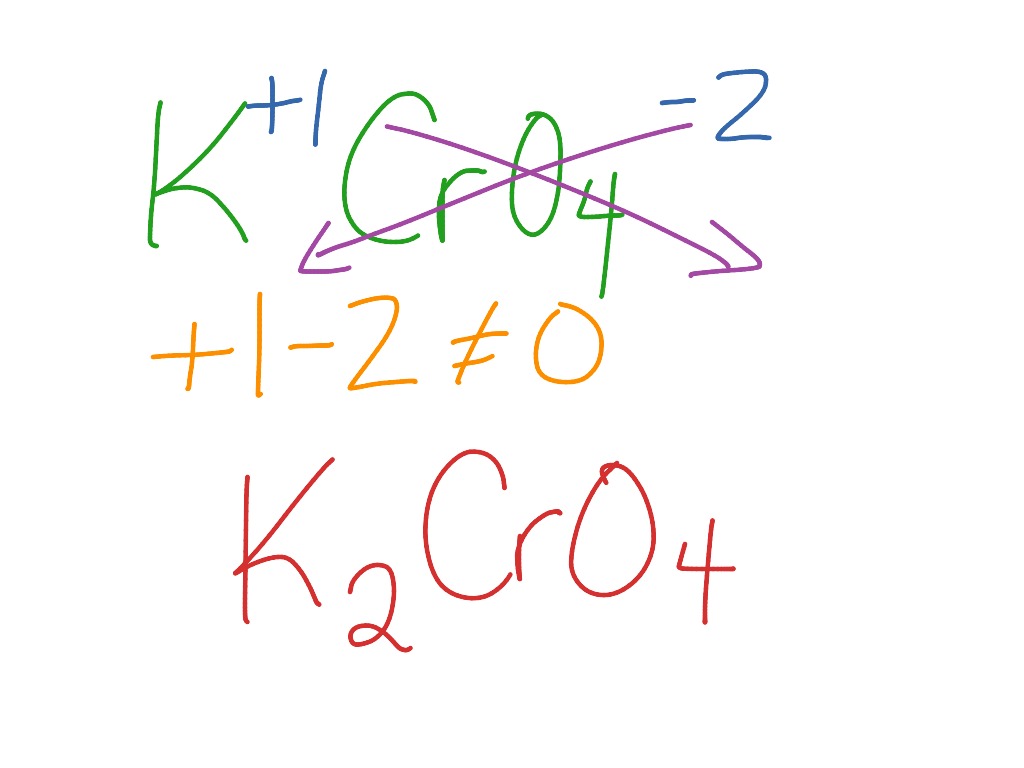
Formula for potassium chromate. This way students can see that the ions combine in whole number ratios in order to produce a neutral chemical species. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. KNO3 potassium nitrate 8.
Give either the name or formula with the correct charge for each of the cations. It is an ionic compound with two potassium ions K and the negatively charged dichromate ion Cr2O7- in which two hexavalent chromium atoms with oxidation state 6 are each attached to three oxygen atoms as well as a bridging oxygen atom. Na2CrO4 sodium chromate 19.
H 3 O ferric. Among them is lópezite. The salt is popular in the laboratory because it is not.
Potassium dichromate K 2 Cr 2 O 7 is a common inorganic chemical reagent most commonly used as an oxidizing agent in various laboratory and industrial applications. The chemical formula for potassium dichromate is K 2 Cr 2 O 7 and the molar mass is calculated to be 294185 gmol. You should complete this by Sunday.
Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound. LiCIO3 lithium chlorate 10. Sodium thiosulfate ____Na2S2O3_____ 3.
Its important to remember that common names are inaccurate and vary from one place and time to another. Give the formula for each compound. The chemical formula calculator also contains the names of a range of.
CdOH2 cadmium hydroxide 13. Ionic Compounds Summary Name the following compounds. Write the Formula Formula Unit for the following Compounds.
All that is left is to convert the yellow potassium chromateVI solution into orange potassium dichromateVI solution. NH42SO4 ammonium sulfate 20. Chromate containing minerals are rare.
This substance is used in the manufacture of dyes and in textile dyeing processes. An alloy of sodium and potassium NaK is used as a heat-transfer medium. Rare potassium chromate minerals and related compounds are found in the Atacama desert.
Use the stock form for the transition metals. Potassium chromate is highly corrosive and is a strong oxidizing agent. NiS nickel II sulfide Write the chemical formula for each of the following compounds.
Potassium chromate primarily affects the nose throat and lungs causing. If there is a common subscript such as 2 as in. MgOH2 magnesium hydroxide 9.
FeNO22 iron II nitrite 9. This reaction is also described further up the page. Cu2CO3 copper I carbonate 8.
Potassium carbonate K 2 CO 3 2. Many potassium salts are of utmost importance including the hydroxide nitrate carbonate chloride chlorate. C 2 O 4 2-Peroxide.
NO 2 barium. PbCO32 lead II carbonate 12. Notes about the Materials The list of ions was based on a dry lab from JA.
S 2 O 3 2-Carbonate. Which is also. Ionic Compounds Naming and Formula Writing.
It is a crystalline ionic solid with a very bright red-orange color. Chromate CrO 4 2-cyanide CN-dichromate Cr 2 O 7 2-dihydrogen phosphate H 2 PO 4 - or H 2 O 4 P-formate CHO 2-or HCOO-or CHOO-hydrogen sulfate or bisulfate HSO 4-hydrogen sulfite or bisulfite HSO 3-hydrogen phosphate HPO 4 2-hydroxide OH-hypochlorite ClO-nitrate NO 3-nitrite NO 2-oxalate C 2 O 4 2-perchlorate ClO 4-permanganate MnO 4-peroxide O. SnCN2 tin II cyanide.
B the chemical formula of the compound appears after the arrow. Chemical or scientific names are used to give an accurate description of a substances composition. Chemical Formula Nomenclature Practice.
31 cobalt III chromate Co2CrO43 32 ammonium oxide NH42O 33 potassium hydroxide KOH 34 lead IV sulfate PbSO42 35 silver cyanide AgCN 36 vanadium V nitride V3N5 37 strontium acetate SrC2H3O22 38 molybdenum sulfate MoSO43 39 platinum II. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions.
Each card has the name in both the old system and the stock system. NH42CrO4 ammonium chromate 17. As with all hexavalent chromium compounds it is acutely and chronically harmful to health.
In the event such products are used goggles should be worn at all times. In its solid form potassium chloride. 2 reduce it to.
Even so you rarely ask someone to pass the sodium chloride at the dinner table. Avoid all of these. Copy this to my account.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. You may remember that that is done by adding acid. At work request a material safety data sheet to help identify alternatives that are safe hence avoiding contact with material containing chromates.
ZnHCO32 zinc bicarbonate 15. This is described above if you have forgotten. Potassium chloride is an ionic salt featuring a bond between an alkali metal and a halogen.
Iron II chloride 18. Potassium chloride is characterized by a colourless crystalline appearance and an odourless smell. Identify the charges Mg.
Compound Name Type of Compound. Sodium sulfate Na 2 SO 4. Potassium Chromate is a yellowish crystalline inorganic compound that emits toxic chromium fumes upon heating.
BaClO42 barium perchlorate 11. Hg2NO22 mercury I nitrite 16. It is denoted by the chemical formula KCl and is made up of potassium cations and chloride anions in a 11 ratio.
Complete these in lab and on your own time for practice. If the subscript is a 1 it does not need to be written. Cr 2 K 2 0 7 the hexavalent form of.
SnSO4 tin II sulfate 14. The solution turns yellow as potassium chromateVI is formed. Parentheses and a subscript are not used unless more than one of a polyatomic ion is present in the formula unit eg calcium sulfate CaSO 4 not CaSO 4.
O 2 2--3 ions. Potassium is an essential constituent for plant growth and is found in most soils. Crocoite PbCrO 4 which can occur as spectacular long red crystals is the most commonly found chromate mineral.
Sodium chloride and potassium chloride. Sodium nitrite NaNO2 31. Cross the Charges Mg.
Sulfur dioxide ____SO2_____ 2. Write Formula Unit For the Below. K2Cr2O7 potassium dichromate 6.
KHCO3 potassium bicarbonate 7. AgNO3 silver nitrate 10. Determining the formula for Magnesium Fluoride.
Unfortunately there is a problem here. Potassium carbonate 2K CO 3 2- K 2 CO 3. Cr 2 O 7 2-Thiosulfate.
Beran - Laboratory Manual for Principles of General Chemistry.
PO 4 3-monohydrogen phosphate. Sodium chromate was also available as a chemically pure grade as the tetrahydrate and in solutions.

Sodium Chromate Tetrahydrate 99 10034 82 9
Ionic Compounds Summary Name the following compounds.

The formula of sodium chromate is. When an aqueous solution of sodium hydroxide NaOH is added to an aqueous solution of chromiumIII nitrate CrNO33 a precipitate of chromiumIII hydroxide CrOH3 forms. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. Cr 2 O 7 2-Thiosulfate.
Potassium carbonate K2CO3 22. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. Approximately 136000 tonnes 150000 tons of hexavalent chromium mainly sodium dichromate were produced in 1985.
Ammonium hydroxide NH4OH 34. The formula unit for the ionic compound sodium dichromate consists of which of the following. Nickel II carbonate NiCO3 24.
C 2 O 4 2-Peroxide. Ce 2 CrO 4. Formula gives the name of all the elements present in the molecule.
That is formula also represents 6022 10 23 molecules of the substance. Chromate and dichromate salts of heavy metals lanthanides and alkaline earth metals are only very slightly soluble in water and are thus used as pigments. Na 3 C 6 H 5 O 7.
Formula also represents one mole of molecules of the substance. 1 Na 2 ion and 1 Cr 2 O 7 2-ion. E-mail to a friend.
NH42SO4 ammonium sulfate 20. Sodium bicarbonate IUPAC name. 26 sodium hydride NaH 27 beryllium hydroxide BeOH2 28 zinc carbonate ZnCO3 29 manganese VII arsenide Mn3As7 30 copper II chlorate CuClO32 31 cobalt III chromate Co2CrO43 32 ammonium oxide NH42O 33 potassium hydroxide KOH 34 lead IV sulfate PbSO42 35 silver cyanide AgCN.
Cs 2 CrO 4. 2 Na ions and 1 Cr 2 O 7 2-ion. Thio-implies replacing an oxygen atom with a sulfur atom.
Cr 2 O 7 2-Anions from Organic Acids. Formula and the correct name of the chemical compound. Similarly calcium chloride would score 4 points two.
Silver chromate is sparingly soluble in aqueous solutions. 4 Na ions and 1 Cr 2 O. Na2CrO4 sodium chromate 19.
CrO 4 2-Hydrogen carbonate or Bicarbonate. O 2 2--3 ions. Use the stock form for the transition metals.
Formula writing rules to write the correct chemical formulas for each compound. You need 2 chloride anions to balance each calcium cation. Write a balanced net ionic equation for this.
Chlorides as sodium chloride 002. Potassium chromate is highly corrosive and is a strong oxidizing agent. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Give the formula for the following. NiS nickel II sulfide Write the chemical formula for each of the following compounds.
Sodium thiosulfate ____Na2S2O3_____ 3. Na 2 CrO 4. Potassium Chromate is a yellowish crystalline inorganic compound that emits toxic chromium fumes upon heating.
Sodium hydrogencarbonate commonly known as baking soda or bicarbonate of soda is a chemical compound with the formula NaHCO 3It is a salt composed of a sodium cation Na and a bicarbonate anion HCO 3 Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powderIt has a slightly salty alkaline taste resembling that of. S 2 O 3 2-Carbonate. Give the formula for each compound.
Cr 2 O 7 2-dichromate. Empirical Formula Hill Notation. C 21 H 33 Li 3 N 7.
Silver sulfide Ag2S 23. Chemical formulas can be. Therefore the formula of calcium chloride is CaCl2.
Aluminum hydroxide AlOH3 33. Complete these in lab and on your own time for practice. The reward for building a correct chemical compound is receiving a number of points based on the number value of the cards played ie.
Similarly in sodium carbonate you have sodium cations Na and carbonate anions CO 3 so. What is the correct formula unit for the ionic compound cesium chromate. This substance is used in the manufacture of dyes and in textile dyeing processes.
1 Na ion and 1 Cr 2 O 7-ion. Potassium chromate primarily affects the nose throat and lungs causing. And water insoluble materials less than 001.
PO 4 3-dihydrogen phosphate. You should complete this by Sunday. Copy this to my account.
Changing between them is easy. HCO 3-hydrogen carbonate bicarbonate HSO 4-hydrogen sulfate bisulfate HSO 3-hydrogen sulfite bisulfite There are some regularities in the names of these polyatomic ions. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.
C 23 H 38 N 7 O 17 P 3 S. 1 Na ion and 1 Cr 2 O 7 2-ion. LiCIO3 lithium chlorate 10.
Vanadium as V less than 0001. Formula represents a definite mass of the substance. Coenzyme A trilithium salt.
You are probably more familiar with the orange dichromateVI ion Cr 2 O 7 2- than the yellow chromateVI ion CrO 4 2-. Ionic Compounds Naming and Formula Writing. Sulfates as sodium sulfate 041.
Chromates and dichromates are used in chrome plating to protect metals from corrosion and to improve paint adhesion. Formula gives the number of atoms of each element present in one molecule. The Ksp of Ag2CrO4 is 112 1012.
A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another. Sulfur dioxide ____SO2_____ 2. Na 2 Cr 2 O 7.
Chemical formula plays an important role in understanding different concepts of chemistry. If you add dilute sulphuric acid to the yellow solution it turns orange. Sodium chloride would score 2 points 1 point for the cation and one for the anion.
C 5 H 4 N 4 O 3. Iron III oxide Fe2O3 32. Acetyl coenzyme A sodium salt.
Cs 2 CrO 4 2. What is the. Chemical Formula Nomenclature Practice.
If you add sodium hydroxide solution to the orange solution it turns yellow. Technical-grade anhydrous sodium chromate available from one USA company has the following typical analysis. An ionic compound is composed of a metal and a non.
The calculator can be used to calculate the chemical formula of a range of 1. Calcium chloride is made of calcium ions with 2 charges Ca and chloride ions with 1 charge only Cl. Compound Name Type of Compound.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. C 2 O 4 2-Hydroxide. Empirical Formula Hill Notation.
Sodium nitrite NaNO2 31.

