The overhead vapors from the column are condensed and collected in an accumulator from which the noncondensables flow to sulfur recovery facilities or are incinerated. Thiols sulfides and thioesters.
14 Thiols And Sulfides Chemistry Libretexts
Start typing to see products you are looking for.

Thiols and sulfides. Below is the structure of diethyl ether a common laboratory solvent and also one of the first compounds to be used as an anesthetic during operations. Peroxides followed by disulfides. The deprotonated forms of alcohols phenols and thiols are called alkoxides phenolates and thiolates respectively.
138 Nomenclature Synthesis and Reactions of Sulfides. 137 Nomenclature Synthesis and Reactions of Thiols. Thiols sulfides 40 20 thiol SH 15 13 thiols sulfides 25 20 50 30 N C with without H amines amides amines 30 23 H S C H N C H Typical 1H and 13.
And in the second step we add a proton source. Sulfides are typically prepared by alkylation of thiols. Alcohols and Phenols followed by thiols selenols 12.
Thiols sulfides 40 20 thiol SH 15 13 thiols sulfides 25 20 50 30 N C with without H amines amides amines 30 23 H S C H N C H Typical 1H and 13. Ethers followed by sulfides selenides 1. Reactions of Alcohols.
Alcohols ethers epoxides sulfides Nomenclature and preparation of epoxides. An sulfur atom between two sp 3-hybridized carbons. Alcohols ethers epoxides sulfides Ring-opening reactions of epoxides.
0 Complete 01 Steps. Such reactions are usually conducted in the presence of a base which converts the thiol into the more nucleophilic thiolate. Synthesis and Reactions of Amines 1 Topic.
Methylation is catalysed by thiopurine methyltransferase in the cytoplasm and thiol methyltransferase in microsomes and both reactions require S-adenosyl-l-methionine as a methyl group donor. Thiols and alcohols are also very different in their reactivity thiols being more easily oxidized than alcohols. A thiol group SH bound to a sp 3-hybridized carbon.
Hydroperoxides followed by thiohydroperoxides selenohydroperoxides 13. Zclassesspectroscopyall spectra tables for webDOC. So in this video well look at the ring opening reactions of epoxides using strong nucleophiles.
143 Interpreting More IR. Females offering pedicure and foot care in a spa salon may also be affected by pitted. Thiolates are more potent nucleophiles than the corresponding alkoxides.
3 Alkanes and Alkane Isomers Alkanes. Conjugated systems and pericyclic reactions. Pitted keratolysis is much more common in males than in females.
Products Chemistry Synthesis Coupling reagents Protecting reagents Fluorination reagents Oxidizing and reducing agents Superdry. 142a IR Spectra of Carbonyl Compounds. Thiols or more specific their conjugate bases are readily alkylated to give sulfides.
RBr HSR RSR HBr. R 3 CLi R 1 SSR 2 R 3 CSR 1 R 2 SLi. Flue gas has also been used as the stripping medium.
A direct difunctionalization protocol of alkenes with nitriles and thiols under metal-free synthesis conditions provides various β-acetamido sulfides with very good yields simply by using inexpensive molecular iodine as a catalyst DMSO as a mild oxidant and readily available thiols as thiolating reagents. Sulfides and mercaptans are removed from wastewater streams by various methods. A protonated alcohol is an oxonium ion.
Chapter 14 IR Spectroscopy and Mass Spectrometry. A halogen atom bound to a carbon atom in an alkyl group X F Cl Br I. Advanced Alkenes and Alkynes Topics 1 Topic.
141 Introduction to IR Spectroscopy. The bad smell is due to sulfur compounds produced by the bacteria. Thiopurine methyltransferase is present in human liver kidney and.
Who gets pitted keratolysis. Alcohols ethers epoxides sulfides. CN CN CN.
Sulfur analogs of alcohols are called thiols or mercaptans and ether analogs are called sulfides. Occupations at risk include. The American Elements solution dilution calculator is a tool to help determine the volume of a solvent required to yield a solution of given volume and concentration molarity of a fixed amount of solute.
The chemical behavior of thiols and sulfides contrasts with that of alcohols and ethers in some important ways. Simple aliphatic and aromatic thiols undergo S-methylation in mammals to produce the corresponding methyl thioether or sulfide. Thiols sulfides disulfides acidic basic HO opsin Lys-NH2 HN H Lys- opsin rhodpsin Carbon-nitrogen multiple bonds N imine Schiff base CCN nitrile cyano group basic CH O CC O Carbonyl-oxygen double bonds carbonyls aldehyde ketones CO O H CO O C carboxylic acid ester CCl O acid chloride CO O C O anhydrides CN O amide acidic.
Some refineries strip the wastewater in a column with live steam. One to six substituents on benzene ring. So in the first step we add a strong nucleophile to our epoxide.
Conjugated systems and pericyclic reactions. How to Convert a Trans Alkene into a Cis. Chem 2016 81.
1 CN. Bottoms water from steam. 139 Organic Synthesis with Ethers and Epoxides.
Should undergo oxidative. 0 Complete 01 Steps. In an ether functional group a central oxygen is bonded to two carbons.
Alcohols ethers epoxides sulfides Thiols and sulfides. If oxides have a lot of ring or angle strain this makes them very reactive towards ring opening. Synthesis and cleavage of ethers.
And the nucleophile is going to end up opening. Zfilesclassesspectroscopytypical spectra chartsDOC C shift. Due to the Thanksgiving Holiday observed in the US All orders placed on Wednesday 24th Thursday 25th Friday November 26th will ship out the week of November 29th.
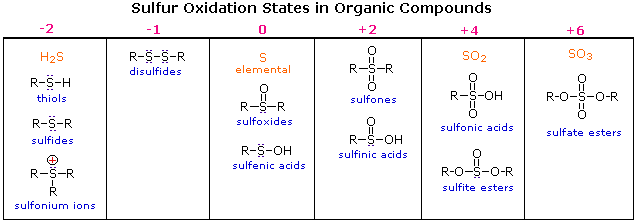
142b The Effect of Conjugation on the Carbonyl Stretching Frequency. Water soluble samples known to contain sulfur as sulfate sulfite or low molecular weight water soluble sulfonic acids RSO 3 H may need no sample preparation but samples known to contain sulfur in other forms such as sulfides S elemental S o polysulfides Sn thiols RSH organic sulfides and disulfides R-S-R and R-S-S-R thiolesters R-CO-SR etc. Consideration of aliphatic aromatic and αβunsaturated sulfides and thiols evaluated by JECFA 53rd 61st 68th and 76th meetings structurally related to substances in FGE08Rev5.
P-C STRETCH P-CH 3 Asymmetrical deformation at 1450-1395 cm-1 Symmetric deformation at 1346-1255 cm-1 Rock at 977-842 cm-1 P-CH 2-CH 3 and P-CH 2-R have deformation band at 1440-1400 cm-1 P-Ar stretch 1130-1090 cm-1 medium in IR weak in Raman P-C stretch 754-634 medium- weak in IR strong in Raman. Reactions of Alkynes. Due to the Thanksgiving Holiday observed in the US All orders placed on Wednesday 24th Thursday 25th Friday November 26th will ship out the week of November 29th.
Since hydrogen sulfide H 2 S is a much stronger acid than water by more than ten million fold we expect and find thiols to be stronger acids than equivalent alcohols and phenols. Alcohols Ethers Epoxides Thiols Sulfides Amines. Analogously the reaction of disulfides with organolithium reagents produces thioethers.
Sulfides Sulfonates Sulfones Sulfonic Acids Sulfoxides SuperDry Solvents Thiocyanates Thiols and Mercaptans Thiophene Thiopyran Thioureas Threonines Tryptophans Tyrosines Ureas Valines Wittig Reagents Search. RSH RBr B RSR HBBr B base. Scientific Opinion on Flavouring Group Evaluation 91 Revision 3 FGE91Rev3.
A carbonyl group in which the carbon atom is bound to a sulfide group and another carbon atom. Erno Pretsch Philippe Buhlmann Martin Badertscher Structure Determination of Organic Compounds Tables of Spectral Data Fourth Revised and Enlarged Edition 123. Organic compounds with.
Reactions of Alkenes.