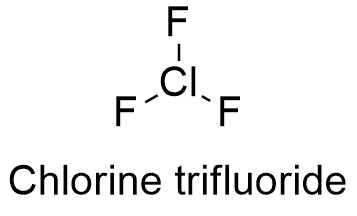
The ligand exhibits hydroxyl proton H a appearing at δ 1568 ppm the aromatic. The IR spectra confirm the potential functional groups of shoot extract responsible for the reduction of Au 3 to gold nanoparticles which exhibit tremendous antibacterial potential of 7631 6847 7985 4810 and 6553 against Escherichia coli Staphylococcus aureus Bacillus pumilus Pseudomonas aeruginosa and Klebsiella pneumoniae respectively.
The complete photoelectron spectra of neutral atoms of two unknown elements.
Ir spectra range for chlorine ions. Enter the email address you signed up with and well email you a reset link. For optimum efficiency the wavelength range is covered simultaneously in three channels - 04-09 micron 09-24 micron and 24-42 micron and the IFS has a 7x7 arcsec FOV to remove slit losses and to acquire absolute photometry on point sources. This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
Experiments have been chosen that have a strong biology component such as Stream Ecology Toxicity Testing and Dissolved Oxygen experiments. In this lab you will perform flame tests of several different metal cations. We would like to show you a description here but the site wont allow us.
Classified as a division 11 explosive if powdered into particles smaller than 15 microns in diameter or if powdered into larger particles but thoroughly dried. The complete photoelectron spectra of neutral atoms of two unknown elements. Gas isotope ratio mass spectrometry or simply IRMS has been the standard tool in areas such as geochemistry environmental sciences 45 and food traceability.
A recent report showed that a porous porphyrin polymer can capture PM ions from the acidic exudate of e-waste 24. 26 Ions are subsequently extracted through an even smaller orifice in the skimmer cone 045 mm diameter and into the main vacuum chamber which is held under high vacuum 7x10 5 1x10 3 Pa by a turbomolecular pump. All the IR data suggest that the metal ions are coordinated to the Schiff base through the phenolic oxygen imino-nitrogen and pyridine nitrogen and with one water molecule.
Mass spectrometry MS is an analytical technique that is used to measure the mass-to-charge ratio of ionsThe results are presented as a mass spectrum a plot of intensity as a function of the mass-to-charge ratioMass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. There are no cold mechanisms facilitating easy and. The maghemite FT-IR spectrum in this range is composed of two wide bands that depending on the particle size and preparation method can be subdivided into more bands.
It is soluble in water and sinks in waterCombustible. The instrument was operated in a positive ion mode using the mz range of 503000. At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2It is colorless odorless non-toxic and highly combustibleHydrogen is the most abundant chemical substance in the universe constituting roughly 75 of all normal matter.
The proton NMR spectra of the free ligand have been recorded in CDCl 3 at room temperature using CCl 4 as an internal standard Figure S2. The analyzed samples were dissolved in methanol. The course will provide laboratory experience in the chemistry of air water and solids.
The complete photoelectron spectra of neutral atoms of two unknown elements. The XPS spectra of GO and N-rGO samples were recorded using the XPS spectrometer K-alpha Thermo Scientific Waltham MA USA where the XPS spectrum for each sample was recorded under pressure conditions 10 8 mbar using a 14866 eV Al K-alpha radiation source from the range of 0 to 1200 eV. At this pressure.
As ions enter this interface region the dramatic reduction in pressure causes a supersonic expansion of the ions generating a so-called free jet. Isotopic percentage and nominal mass calculation are. Crystallinity and interlayer distance.
Ammonium perchlorate appears as a white crystalline solid or powder. Flame Tests of Metal Cations. In this lab how do the metal cations become excited.
Consists in part of K. Object acquisition and guiding is done with an external cryostat-mounted 3 arcmin FOV CCD. Wavelength values here are given for the mid- range of the color indicated.
The characteristic colors observed are due to emitted electromagnetic radiation from the excited metal cations. Gold nanoparticles showed. Hydrogen is the lightest element.
The X-ray diffraction XRD patterns were recorded for. High resolution electrospray ionization mass spectra ESI-HRMS were obtained on a Bruker microTOF spectrometer equipped with an ESI source. Smith and colleagues used.
The IR absorption bands in the region between 200 and 900 cm 1 refer to the vibration of the Fe-O bond and they are different for maghemite and magnetite. Hydrogen is the chemical element with the symbol H and atomic number 1. Chloroacetic acid solid is a colorless to light-brown crystalline material.
Characteristic X-ray spectra are produced. Pembuatan Senyawa kompleks asetial asetanoat. It is transported as a molten liquid and therefore can cause thermal burns.
OR It is the measurement of electromagnetic radiation EMR absorbed or emitted when molecule or ions or atoms of a sample move from one energy state to another energy state. Spectroscopy is the most powerful tool available for the study of atomic molecular structure and is used in the analysis of a wide range of samples. These spe ctra con-sist of several components th e most commo n being K.
For commercial alkaline water electrolyzers the basic electricity demand is 43573 kWh for yielding 1 m 3 of H 2 at the cell voltages of 18 24 V and practical current level of 300500. In contrast to organic mass spectrometers MS that yield structural information by scanning a mass range over several hundred Dalton for characteristic fragment ions IRMS instruments Figure 2 achieve highly accurate and. First the fundamental notions of mass spectrometry are explained so that the reader can easily cover this chapter graphs main pick molecular ion illogical pick nitrogen rule etc.
The most intense peak in the isotopic pattern is noted. The chapter includes an introduction to the main ionisation techniques in mass spectrometry and the way the resulting fragments can be analysed. The suggested laboratory experiments will consist of a broad range of scientific inquiry that will enhance the lecture material covered in CHEM 020.
The two naturally occurring isotopes of chlorine are chlorine-35 and chlorine-37. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N.

Making An Atom Model 10 Would An Atom Like Chlorine With A Different Number Atom Model Bohr Model Atom Model Project
Chlorine-37 is composed of 17 protons 20 neutrons and 17 electrons.

Number of neutrons for chlorine. One may also ask do most chlorine atoms have 18 neutrons or 20 neutrons. For example chlorine Cl deserve to be shown as. Subtracting 17 from 35 equals 18 which is the number of neutrons in a chlorine atom.
_612 extrmC _613 extrmC and _614. Chlorine has 17 protons in its nucleus and its most common isotope has 18 neutrons. One of them has 18 neutrons the other has 20.
A chlorine atom has a mass number of 35. What is the mass of a chlorine atom. How are the number of electrons and neutrons of an atom determined.
Not all atoms of an element therefore have the same atomic mass number. Atoms of chlorine with. Including or eradicating a neutron from an atoms nucleus creates isotopes of a selected component.
So if you have a Periodic Table you would see that chlorines atomic number is 17. These isotopes occur naturally with different abundances. Therefore a neutral atom of chlorine contains 17 protons and 17 electrons.
Chlorine-37 contains 20 neutronsTime for an atom lesson. Only two chlorine isotopes exist in significant amounts in nature. Trace amounts of radioactive 36 Cl exist in the environment in a ratio of about 710 13 to 1 with stable isotopes.
23 10 protons 13 number of neutrons in the atom. Name Chlorine Atomic Mass 35453 atomic mass units Number of Protons 17 Number of Neutrons 18 Number of Electrons 17. Every chlorine atom has1 7 electrons 2 17 neutrons 3 a mass number of 35 4 an atomic number of 17 None.
How many protons neutrons and electrons are there in a neutral atom of the isotope of chlorine named chlorine 35. _612textrmC _613textrmC and _614textrmC. Each isotope of an element is characterized by an atomic number total number of protons a mass number total number of protons and neutrons and an atomic weight mass of atom in atomic mass units.
If we look up chlorines atomic mass number on the periodic table it is 35 which indicates the sum of protons and neutrons in the nucleus. Now To get the number of neutrons in a Chlorine atom look at its atomic mass which is 35453 rounded to 35 and the number of protons in Chlorine is 17. An atom of chlorine-35 incorporates 18 neutrons 17 protons 18 neutrons 35 particles within the nucleus whereas an atom of chlorine-37 incorporates 20 neutrons 17 protons 20 neutrons 37 particles within the nucleus.
That means that the total mass of a chlorine atom is about 355 atomic mass units. In the top left-hand corner of the box of Cl you see the number 17 which is the atomic number for chlorine. However some chlorine atoms have 18 neutrons while other chlorine atoms have 20 neutronsAtoms of chlorine with 18 and 20 neutrons are called chlorine isotopes.
However an elements mass numbers can vary which means that it can have different numbers of neutrons. Neutron Number and Mass Number of Chlorine Mass numbers of typical isotopes of Chlorine are 35. Why does this indicate that most chlorine atoms contain 18 neutrons.
Variations of an element with different numbers of neutrons are called isotopes. Then all we need to do is a little subtraction 37-17 to find the number of neutrons 20. The amount of protons will.
Chlorine-36 is composed of 17 protons 19 neutrons and 17 electrons. Chlorine-35 is composed of 17 protons 18 neutrons and 17 electrons. This means that chlorine has 17 protons in its nucleus both for the regular atom and for the isotope of chlorine.
An atom of chlorine-35 contains 18 neutrons 17 protons 18 neutrons 35 particles in the nucleus while an atom of chlorine-37 contains 20 neutrons 17. The chlorine isotope has 17 protons and 20 neutrons. All atoms of chlorine Cl have 17 protons but there are chlorine isotopes with 15 to 23 neutrons.
All chlorine atoms for instance have 17 protons. Mass number and atomic number are two important pieces of information about a nucleus A nucleus can be represented using the symbol notationWhereA is the mass numberZ is the atomic numberX is the chemical symbol of the elementA - Z number of neutrons NFor example chlorine Cl can be shown asCarbon has three isotopes. Those with 18 neutrons 7553 of all chlorine atoms found in nature and those with 20 neutrons 2447.
In the case of chlorine some atoms have 18 neutrons this isotope is called Cl-35 because the mass is 35 some have 20 neutrons isotope Cl-37 and a few have different number of neutrons. However some chlorine atoms have 18 neutrons while other chlorine atoms have 20 neutrons. Hence the number of neutrons in Chlorine atom 35 17 18.
All chlorine atoms for instance have 17 protons. There are two fairly common isotopes for chlorine. Carbon has actually three isotopes.
In this video well use the Periodic table and a few simple rules to find the protons electrons and neutrons for the element Chlorine Cl. Since we used the average atomic mass to calculate the number of neutrons this is why we can conclude that most Chlorine atoms contain 18. Chlorine is number 17 on the Periodic Table which means it has 17 protons.
Chlorine has 17 protons in its nucleus and its most common isotope has 18 neutrons. So although chlorine has a mass number of 35 which means it has 18 neutrons it can also have a mass number of 37 which means it has 20 neutrons.
After turning red both papers are then bleached white. In this video well write the formula for Chlorine gas.
In addition we sell it as a liquefied compressed gas.

Formula for chlorine gas. The combination of bleach sodium hypochlorite with acid produces chlorine gas a heavy green-yellow gas with a strong odor. It is yellow-green in colour and has an odour which is similar to to the household bleach. For Chlorine gas we need to know that there are two Cl atoms bonded in gases like Oxygen gas O 2 Fluorine gas F 2 Bromine gas Nitrogen gas.
Chlorine gas has also been used as an industrial solvent and has other industrial uses such as the production of bulk materials bleached paper products plastics such as PVC and solvents. Preparation of chlorine gas. It is an extremely reactive element and a strong oxidising agent.
It is greenish-yellow with a pungent suffocating odor gas. Cl2 chlorine never exist in nature but if chlorine is produce in chemical reactions it will bond to each other like oxygen and nitrogen to form a diatomic molecule. Physical properties of chlorine gas.
HCl MnO 2 MnCl 2 Cl 2 2H 2 O. Preparation of chlorine gas. We can prepare chlorine by combining hydrochloric acid and manganese dioxide.
7 The chemical formula for chlorine is Cl 2 and its molecular weight is 7090 gmol. The chemical structure of chlorine gas is Cl2 and its molecular weight is 70 gmol. Chlorine gas Cl2 - Chlorine is the chemical element with the formula Cl2 commonly used as disinfectant.
2 Chlorine gas has a pungent choking smell. Among the elements it has the highest electron. The reaction just shows the elements in their standard states their phases at room temperature at 1atm pressure for example chlorine is a gas at room temperature Cl2 bromine is a liquid.
Chlorine gas is an inorganic gas that is utilised in controlled quantities in the chemical industry. Chlorine is an element. Study it and answer the questions that follow.
In addition we sell it as a liquefied compressed gas. Its molar mass is 70906 grams per mole. An explanation of how to balance and write the equation for Sodium Chlorine gas and the correct coefficients for each substance in the equationTo balance.
HCl MnO 2 MnCl 2 Cl 2 2H 2 O. Reactive oxygen species ROS such as superoxide O 2 hydrogen peroxide H 2 O 2 and potentially hydroxyl radical can be formed both via recruited. Ii The equation for the redox reaction that takes place is MnO 2s 4 HCl aq MnCl 2 aq 2H 2 O l Cl 2 g Explain using oxidation.
Chemical compound of the family of halogens chlorine is a yellowish-green gas with suffocating smell very unpleasant and it is extremely toxic Chlorine 17Cl. Chlorine gas is an inorganic gas that is utilised in controlled quantities in the chemical industry. Also it converts to liquid at 35 o C and is slightly soluble in water.
3 Chlorine gas turns moist litmus paper from blue to red and moist universal indicator paper to red - it is acidic. The odor threshold is 031 ppm. What is the chemical formula for chlorine gas.
I Complete the set up to show how dry chloride gas may be collected. Also it converts to liquid at 35 o C and is slightly soluble in water. It is greenish-yellow with a pungent suffocating odor gas.
4 Chlorine gas will put out a lit splint. Hint for Writing the Formula for Chlorine gas. The chlorine gas formula is Cl 2.
What are the General Tests for Chlorine Gas. Chlorine gas phase is the result of combining two chlorine Cl atoms or the formula Cl 2. Chlorine is a yellow-green gas at room temperature.
Visit BYJUS to understand the properties structure and its uses. Its diatomic molecular form is indicated by the symbol Cl2. For Chlorine gas use the hints and resources below to help write the formula.
The chemical formula of chlorine gas is Cl 2. These reactions show the direct combination of the elements chlorine gas and sodium to form NaCl. Hydration of chlorine gas Cl 2 leads to formation of HCl and HOCl hypochlorous acidAs indicated both Cl 2 and HOCl can react with airway lining constituents.
Chlorine Cl2 is NOT the same as the chloride ion Cl-. It is soluble in water and reacts to form hypochlorous acid and hydrochloric acid. Mgm 3 ppm molecular weight of the.
Chlorine is a chemical element with the symbol Cl and atomic number 17. We can prepare chlorine by combining hydrochloric acid and manganese dioxide. To convert concentrations in air at 25C from ppm to mgm 3.
It exists as a yellow-green gas at standard temperature and pressure and its density is 32 grams per liter. Structure properties spectra suppliers and links for. 1 Chlorine gas Cl 2 g is green-yellow in colour.
Physical properties of chlorine gas. Postulated mechanisms for airways injury due to chlorine inhalation. Chlorine gas is an inorganic gas polemic for being used as chemical weapons it is mostly used in controlled quantities in chemical industry.
4 Chlorine has a suffocating odor. Chlorine is a greenish-yellow gas that is slightly soluble in water. Note that Chlorine gas is one of the seven major diatomic gasesThe seven major diatomic elements in.
The chemical formula for chlorine gas is Cl 2. The second-lightest of the halogens it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them.
Chlorate when followed by a Roman numeral in parentheses eg. In 2020 about 38 of the countrys salt consumption was used to produce chlorine sodium hydroxide synthetic soda ash and other chemicals.
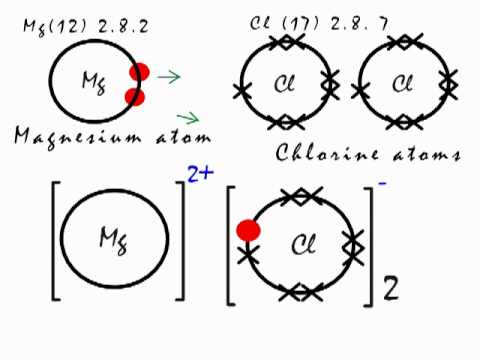
Ionic Bonding In Mgcl2 Magnesium Chloride Youtube
The nitrogen and oxygen which makes up the bulk of the atmosphere also exhibits covalent bonding in forming diatomic molecules.
.jpg)
Magnesium and chlorine. Its well absorbed in. The chlorate anion has the formula ClO 3In this case the chlorine atom is in the 5 oxidation state. For Swimming Pool or Spa.
Chlorates are the salts of chloric acid. Express the number of moles of Mg Cl and O atoms numerically separated by commas. Micronutrients also called trace elements are also essential but they are only required in very small amounts.
From a thermodynamic standpoint chlorine is most. Instead of producing their salt by traditional mining methods the chemical industry obtains much of their salt by. You can see in the diagram below that there are seven electrons in the outermost circle.
It encourages learners to develop confidence in and a positive attitude towards chemistry and to recognise its importance in their own lives and to society. The halogens such as chlorine also exist as diatomic gases by forming covalent bonds. In thermal production dolomite is calcined to magnesium oxide MgO and lime CaO and.
View User Profile Send Message Posted Nov 26 2021 507. Dissolved Oxygen Formaldehyde Fluoride Glycol Hardness Hydrazine Hydrogen Peroxide Hypochlorite Iodide Iodine Iron. - helps chemist understanding how.
I think this mod is discontinued since its not updating on the source page. Magnesium chloride is a magnesium salt that includes chlorine an unstable element that binds well with other elements including sodium and magnesium to form salts. Index Bond concepts Chemical concepts.
Chlorine for example has seven electrons in its outer electron shell. Electron Configuration Notation-shows the arrangment of electrons around the nucleus of an atom. Comparison of ionic and covalent materials.
In contrast energy would be required to add a second electron to a chlorine atom. Milliard Chlorine Floater Floating Chlorine Dispenser Large Capacity and Adjustable Release Fits 1-3in. In a picture the valence electrons are the ones in the outermost shell.
In reply to. Covalent bonding can be visualized with the aid of Lewis diagrams. These elements effect growth reproduction and the effect that.
Chlorine has 7 valence electrons. We would like to show you a description here but the site wont allow us. Power consumption is 12 to 18 kilowatt-hours per kilogram of magnesium produced.
Magnesium is very chemically active it takes the place of hydrogen in boiling water and a great number of metals can be produced by thermic reduction of its salts and oxidized forms with magnesium. Lead Lux Magnesium Manganese Molybdenum Nickel Nitrate Nitrite Nitrogen ORP Ozone. PH Phosphate Phosphorus Potassium Refractometry Relative Humidity.
Magnesium and nitrogen also play a role in the production of chlorophyll. Browse CurseForge App Create a Project Feedback and News Idea Suggestion Portal. How do i calculate the number of moles of magnesium chlorine and oxygen atoms in 770 moles of magnesium perchlorate MgClO_4_2.
The electron configuration of chlorine is 1s2 2s2 2p6 3s2 3p5 or Ne3s2 3p5. The third period contains eight elements. Magnesium is an effective flocculant and very efficient in improving water clarity and can reduce the need for backwashing.
Coordinate responsibilities and prepare to enter the scene as part of the. The chlorine can be reused in the dehydration process. Chlorate VII refers to a particular oxyanion of chlorine.
Between 50 and 100. Chlorate can also refer to chemical compounds containing this anion. Carbon Dioxide Chloride Chlorine Chlorine Dioxide Chromium COD Colour Conductivity TDS Copper Cyanide Cyanuric Acid.
The first two sodium and magnesium are members of the s-block of the periodic table while the others are members of the p-block. 47 out of 5 stars 2884. The grinder wheels are available in INOX-Prime Plus a blend of premium aluminum oxide and white aluminum oxide and Zirconia Prime high-performance zirconia.
Consult with the Incident Commander regarding the agent dispersed dissemination method level of PPE required location geographic complications if any and the approximate number of remains. Magnesium reacts only slightly or not at all with most of the alkalis and many organic substances like hydrocarbons aldehides. FREE Shipping on orders over 25 shipped by Amazon.
Weldcote a division of Zika Group has introduced a new line of right-angle grinder wheels for use on high-quality steelDesigned in Japan and manufactured in Thailand the wheels are free from iron sulfur and chlorine contaminants. It joins together with most non-metals and almost every acid. Chlorine and other gases are generated at the graphite anodes and molten magnesium metal floats to the top of the salt bath where it is collected.
The magnesium blend has a water softening effect that assists skin hydration improves skin barrier function and reduces skin roughness and inflammation. Magnesium Mg Aluminum Al Silicon Si Phosphorus P Sulfur S Chlorine Cl Argon Ar Potassium K Calcium Ca Chromium Cr Cr 2 Cr 3 Copper Cu Cu Cu 2 Iron Fe Fe 2 Fe 3 Read my article in Science Education based on my dissertation. - can be written using the period table or.
Choose the right micronutrients. - helps chemist understanding how elements form chemical bonds. The chemicals were then used as ingredients in plastics paints solvents and a variety of household and industrial cleaning products.
Chlorine readily bonds with other elements so that it can have a filled energy level like argon. Get it as soon as Wed Dec 8. Electron Configuration Notation-shows the arrangment of electrons around the nucleus of an atom.
All of the period 3 elements occur in nature and have at least one stable isotope. Chlorine a forge port of Sodium. Chlorine Cl Argon Ar Potassium K Calcium Ca Chromium Cr Cr 2 Cr 3 Copper Cu Cu Cu 2 Iron Fe Fe 2 Fe 3 Read my article in Science Education based on my dissertation.
Use Magnesium it is more stable and has more compatibility. Between 30 and 50. Calcium is a part of the make-up of cell walls and plays a role in the growth of cells.
Sodium magnesium aluminium silicon phosphorus sulfur chlorine and argon. The 3s2 3p5 electrons are the outermost electrons so chlorine has seven valence electrons. It is also estimated that there are.
3288 kJ per mole of chlorine atoms are released when chlorine acquires a single electron. Chlorine exposure may result in chronic inflammation of the large airways bronchitis. As predicted by valence shell electron pair.
Chlorine is in group 17 of periodic table also called the halogens and is not found as the element in nature - only as a compound. Our ASA level Chemistry specification provides a broad coherent satisfying and worthwhile course of study. By This Old House Earns.
The most common of these being salt or sodium chloride and the potassium compounds sylvite or potassium chloride and carnallite potassium magnesium chloride hexahydrate.
This OH is a. The Occupational Safety and Health Administration OSHA promulgated its permissible exposure limits PEL of 1 milligram per cubic meter of air mgm 3 for chlorodiphenyl products containing 42 chlorine and 05 mgm 3 for chlorodiphenyl products containing 54 chlorine determined as 8-hour time-weighted average TWA concentrations 35 based on the 1968 Threshold Limit Values TLVs of the.

Iarc Publications Website Chlorinated Drinking Water Chlorination By Products Some Other Halogenated Compounds Cobalt And Cobalt Compounds
Chlorine gas is the least expensive form of chlorine to use.

Halogenated carcinogens chlorine. Dioxin can get into drinking water from. Halogenated hydrocarbons also known as halocarbons are hydrocarbon compounds in which at least one hydrogen atom is replaced by a halogen Group VII A of the Periodic Table atom such as fluorine chlorine or bromineCommon examples of halogenated hydrocarbons include. Inhalation studies with both humans and rats have shown that dichloropropene DCP is readily absorbed conjugated with glutathione via glutathione S-transferase GST and rapidly excreted in the urine as N-acetyl-S-cis-3-chloroprop-2-enyl-cysteine 3CNAC a mercapturic acid metabolite.
As well as the halogenated product. HFRs are persistent bioaccumulative toxins meaning that they accumulate in organisms and the broader environment often reaching alarmingly high concentrations as they travel up the food chain. INPUT CAPACITY NO X EMISSION LIMIT gGJ AND PPM AT 3 O 2 105 TO 105 GJh 10 TO 100 MILLION Btuh.
Halogenated Fire Retardants HFRs are a broad class of flame retardants containing chlorine or bromine that have aroused concern due to their exponential accumulation in human beings in recent years. Chlorine bleaching of pulp and paper and other industrial processes can create small quantities of dioxins in the environment. Carcinogens summary report on the evaluation of short-term in vivo tests EHC 109 1990 Chlordane EHC 34 1984 Chlordecone EHC 43 1984 Chlordimeform EHC 199 1998 Chlorendic acid and anhydride EHC 185 1996 Chlorinated Paraffins EHC 181 1996 Chlorine and hydrogen chloride EHC 21 1982 Chloroalkyl ethers selected EHC 201 1998 Chlorobenzenes other than hexachlorobenzene.
No information is available on the carcinogenic effects of chlorine in humans from inhalation. Rotation around this carbon-carbon bond is possible and does not result in different isomer conformations. Note also that each carbon involved in the double bond is also attached to two different atoms a hydrogen and a chlorine.
A study of six male volunteers exposed at 1 ppm commercial Telone II 506 cis-isomer 452 trans. Several halogenated products derived from methane and ethane CH 3 CH 3 are listed in Table 76 along with some of their uses. Some attention is given to an examination of the potential health effects related to the use of these adsorbents but detailed toxicological.
1-bromopropane C 3 H 7 Br methylene chloride CH 2 Cl 2 chloroform CHCl 3 tetrachloroethylene C 2 Cl 4 and. Many bleaches have broad spectrum bactericidal properties making them useful for. Purchase Encyclopedia of Toxicology - 3rd Edition.
CCME NO x Emission Guidelines for New Boilers and Heaters. In the lower diagram the halogenated alkene has restricted rotation around the double bond. Chronic long-term exposure to chlorine gas in workers has resulted in respiratory effects including eye and throat irritation and airflow obstruction.
Appendix IV to Part 268 Wastes Excluded From Lab Packs Under the Alternative Treatment Standards of 26842c Appendix V to Part 268 Reserved Appendix VI to Part 268 Recommended Technologies To Achieve Deactivation of Characteristics in Section 26842. Deposition from air to. Chlorine is a commonly used household cleaner and disinfectant.
Haloalkanes behave as the R synthon and readily react with nucleophiles. List of Halogenated Organic Compounds Regulated Under 26832. Hydrolysis a reaction in which water breaks a bond is a good example of the nucleophilic nature of haloalkanes.
Print Book E-Book. If the chlorine liquid is released from its container it will quickly return back to its gas state. In the upper figure the halogenated alkane is shown.
Chlorine is a potent irritant to the eyes the upper respiratory tract and lungs. This chapter contains the findings of the Subcommittee on Adsorption of the National Research Councils Safe Drinking Water Committee which studied the efficacy of granular activated carbon GAC and related adsorbents in the treatment of drinking water. The typical amount of chlorine gas required for water.
Chlorine gas is sold as a compressed liquid which is amber in color. Cigarette smoke also contains small amounts of dioxins. For example methane CH 4 can react with chlorine Cl 2 replacing one two three or all four hydrogen atoms with Cl atoms.
The polar bond attracts a hydroxide ion OH NaOH aq being a common source of this ion. Appendix VII to Part 268 LDR Effective Dates of Surface Disposed. It often refers specifically to a dilute solution of sodium hypochlorite also called liquid bleach.
Chlorine as a liquid is heavier more dense than water. Compounds in fuel or combustion air containing halogens chlorine fluorine bromine and iodine Potential carcinogens global warming. Potential carcinogens Halogenated compounds.
Air emissions from waste incineration and other combustion with subsequent deposition to lakes and reservoirs. As a result of their extensive use and persistence PCBs remain ubiquitous environmental contaminants. Up to 24 000 Table 3.
Only haloalkanes that contain chlorine bromine. PCBs were used in cutting oils lubricants and as electrical insulators. Bleach is the generic name for any chemical product that is used industrially and domestically to remove color from a fabric or fiber or to clean or to remove stains in a process called bleaching.
PCBs are a class of synthetic persistent lipophilic halogenated aromatic compounds that were widely used in industrial and consumer products for decades before their production was banned in the late 1970s.