PTWI expressed as Sn including stannous chloride. Iron Fe and water Iron and water.
Answered Iron Iii Chloride Is Reduced By Bartleby
Drinking water may not contain more than 200 ppb of iron.

Iron chloride and tin reaction. Exploding a tin can using methane. Seawater contains approximately 1-3 ppb of iron. To be sure reaction was complete small excess of the SnCl 2 is added and then solution is treated with mercury II chloride.
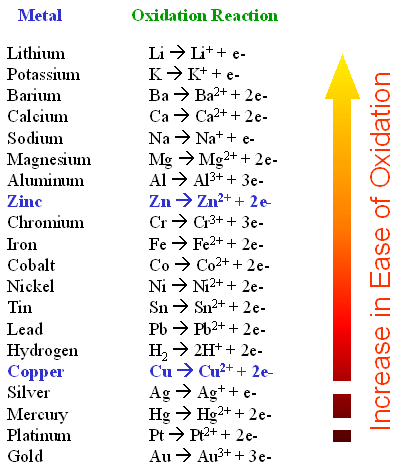
Iron nails Copper foil 2 inch by 18 inch or copper wire Zinc foil 2 inch by 18 inch Aluminum foil 2 inch by 18 inch Tin foil 2 inch by 18 inch Magnesium ribbon 2 inch Magnesium ribbon 1 inch piece 01 phenolphthalein 01 g to 50-50 water-alcohol mixture 01 M potassium ferricyanide K. Iron metal is more electropositive than copper and therefore will displace it from its salts. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website.
When metallic iron oxidation state 0 is placed in a solution of hydrochloric acid ironII chloride is formed with release of hydrogen gas by the reaction Fe 0 2 H Fe 2 H 2. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. It is corrosive to metals and tissue.
Ferrous chloride is a greenish white. The third class contains metals such as chromium iron tin and lead which react only with strong acids. Create a small explosion in this demonstration by electrolysing water to produce hydrogen.
Most algae contain. Iron chloride FeCl2 More. Reaction mechanisms environmental impact and health effects.
The primary aim of the cards is to promote the safe use of chemicals in the workplace. Tin is a post-transition metal in group 14 of the periodic table. The 1988 PTWI was not reconsidered and was maintained at the fifty-fifth meeting 2000.
Click to see our best Video content. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic structure. 2FeCl 3 SnCl 2 2FeCl 2 SnCl 4.
The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and. TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in.
In association with Nuffield Foundation. When iron metal is exposed to air and water usually it turns into rust a. The acute toxicity of tin was assessed at the fifty-fifth meeting but data were insufficient for establishing an acute reference dose.
Use this demonstration to illustrate how methane can create an explosive mixture with the oxygen in air. Simple S N 2 reaction. Tin-plated tiffin boxes are utilised because they.
Tin is non-toxic and its reactivity is lower than that of iron. Properties of the element. In association with Nuffield Foundation.
Rusting of iron can be prevented by tinning. S N 1 and S N 2. Ferrous chloride solution is the greenish-white crystalline solid dissolved in water.
It is obtained chiefly from the mineral cassiterite which contains tin dioxide. Tin plating is usually used for making tin cans as tin is very expensive. The first alloy used on a large scale was.
The Committee reiterated the conclusion reached in 1988 that tin concentrations as low as 150 mgkg in canned beverages. Tin is nontoxic ductile malleable and adapted to all kinds of cold-working such as rolling spinning and extrusionThe colour of pure tin is retained during exposure because a thin invisible protective film of stannic oxide is formed spontaneously by reaction with the oxygen of the air. The chemical symbol for Tin is Sn.
Rivers contain approximately 05-1 ppm of iron and groundwater contains 100 ppm. Food cans are tinned which implies that they have a thin layer of tin on them. Allyl Chloride with HS S N 2 Reaction.
This is our newest publication and has been created to support the school technician profession in Scotland. Take A Sneak Peak At The Movies Coming Out This Week 812 Lin-Manuel Miranda is a Broadway and Hollywood Powerhouse. Metals in the fourth class are so unreactive they are essentially inert at room temperature.
Why does the reaction of iron and copper chloride occur Why does the reaction of iron and copper chloride occur. These metals are ideal for making jewelry or coins because they do. The ironII hydroxide is then further oxidised by oxygen to form hydrated iron.
Why does the reaction of iron and copper chloride occur. It also contains even less active metals such as copper which only dissolves when treated with acids that can oxidize the metal. As a result when an electroplated thin coating of tin metal is deposited on iron and steel items the iron and steel objects are protected from rusting.
2 o Benzyl Chloride with HS S N 2 Mechanism. S N 2 Reaction. It is used in dyeing in medicine and sewage treatment.
The low melting point of tin and its firm adhesion to clean surfaces of. Includes kit list and safety instructions. This is done by dipping the iron into molten tin or by electroplating an iron sheet using tinIV chloride as the electrolyte.
The amount varies strongly and is different in the Atlantic and the Pacific Ocean. It is possible to reduce iron 3 using Jones reductor column filled with granules of amalgamated zinc however most commonly reduction is done with tin II chloride. Stability and structure of carbocations.
S N 2 examples. Substrate structure controls substitution mechanism S N 1 or S N 2. Exploding bubbles of hydrogen and oxygen.
Thus the overall redox reaction is as follows. Fe 0 Cu 2 Fe 2 Cu 0. Benzyl Chloride with HS S N 2 Reaction.
After tin plating the inside of the can is coated with a thin.
Copper is a soft malleable and ductile metal with very high thermal and electrical conductivity. ZnO 2 CCH 3 2.

Solved 761 Zinc Metal Reacts With Aqueous Copper Ii Chegg Com
Zinc is a hidden star.

Copper chloride and zinc reaction. Or until color well developed. Zinc and Copper Chloride Reaction Zn CuSO4 -- Cu ZnSO4. Copper is a chemical element with atomic number 29 which means there are 29 protons and 29 electrons in the atomic structure.
Answer 1 of 17. S3 These are the products. Reducing Cu2 ions with Zinc Metal In Part V zinc metal Zn is added to the copper solution to convert the copper ions back to copper metal Cus.
Zinc salts cause a milky turbidity in water in higher concentrations. By observing this reaction and its products and noting the difference in reactivity between zinc and copper students can familiarise themselves with the idea of competiton reactions. The first organocopper compound the explosive copperI acetylide Cu 2 C 2 Cu-CC-Cu was synthesized by Rudolf Christian Böttger.
Copper is used. CopperII oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Characterization of the synthesized materials was carried out using XRD STEM HRTEM SAED and EDX elemental mapping.
The ameliorating effects of copper2 and sulfate2- ions on concurrent selenite toxicity were compared in two factorial experiments in weanling rats. Additionally zinc may add an unwanted flavour to water. If 20 grams of zinc reacts with hydrochloric acid how much zinc chloride is produced.
Copper will not react with hydrochloric acid. The zinc and copper switch. This reaction is a single Metal replacement reaction.
Shown to be effective in maintaining growth of children with cows-milk protein allergy when used as the primary source of calories. A piece of zinc reacts completely with hydrochloric acid HClaq to produce an aqueous solution of zinc chloride ZnCl2aq and hydrogen gas. Why and why does this reaction even involve electron.
They are reagents in organic chemistry. Cyanide chemistry is comprehensively reviewed by Guo et al. For example if a piece of zinc metal is immersed into a solution containing copper II ions zinc will be oxidized by the copper II ions.
In general catalytic action is a chemical reaction between the catalyst and a reactant. The experiment compares reactivity of three metals. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions.
Sphalerite rejection from copper concentrates is often of major concern as zinc is a penalty element in copper smelting. It is a soft malleable and ductile metal with very high thermal and electrical conductivityA freshly exposed surface of pure copper has a pinkish-orange colorCopper is used as a conductor of heat and electricity as a building material and as a constituent of various metal alloys such as sterling. Following pretreatment with zinc chloride hepatocytes were treated with 20 or 40 uM silver nitrate for 24 hr and cytotoxicity was then assessed by enzyme leakage and loss of intracellular potassium.
Cuprum and atomic number 29. For this type of reaction to occur the reduction potential of the reactant receiving the electrons must be lower than the reduction potential of the reactant giving up electrons. Catalyst in chemistry any substance that increases the rate of a reaction without itself being consumed.
Were rarely aware of it unlike its flashier neighbours in the period table but zinc is a workhorse element that helps us all. This reaction releases hydrogen which reacts with oxygen explosively. Red - semi-matt for Copper Copper Plate Ingredients A Cupric Sulfate.
Visible evidence of this reaction is observed as bubbles of gas being released from the solution. Copper is a chemical element with the symbol Cu from Latin. We have seen this reaction before in the copper chloride labCuH 2 O 6 2 aq Zn s -- Cu s Zn 2 aq 6 H 2 O aq At the same time some of the zinc metal which is present in excess reduces hydronium ions to H 2Zn s 2 H 3 O aq -- Zn 2 aq H 2 g 2 H 2 O l Procedure I wont go over the procedure in step-by-step detail but I will stress some points of.
The solution becomes bluish as some amount of copper is developed. Use the data shown below to determine the enthalpy of reaction per mole of zinc. This is indicated by colour change from blue to colourless.
Remove to hot water while ammonium chloride is added to the cupric sulfate solution. A copper wire is then dipped in it and kept undisturbed for some time. The chemicals Indium chloride tetrahydrate InCl 3 4H 2 O thioacetamide C 2 H 5 NS and Zinc dichloride ZnCl 2 were used.
So here on adding zinc to CuSO4 solution zinc displaces copper from copper sulphate forms zinc sulphate solution. When the pH is fairly. A freshly exposed surface of pure copper has a reddish-orange color.
1 100 free amino acids as nitrogen source. Clinically documented to be hypoallergenic 1 to virtually eliminate the potential for an allergic reaction to the formula in multiple-food-allergic children. In the below reaction the copper metal displaces silver from Silver Nitrate solutionCu s 2AgNo 3aq 2Ag s CuNO 3 2aq Double.
In 2 S 3 and ZnIn 2 S 4 were synthesized using the same. The chemical symbol for Copper is Cu. With zinc also appearing in anti-dandruff shampoos in the form of zinc pyrithione and in underarm deodorants as zinc chloride this is an element that even makes us more attractive to the opposite sex.
Explanation - Zinc is more reactive than copper. The net reaction is Zns2Haq -Zn2aqH_2g The Cl- ions are spectators - they dont change. The solubility of zinc depends on temperature and pH of the water in question.
Solubility of zinc and zinc compounds. Zn 3 PO 4 2. 1L B Ammonium Chloride.
33 of fat blend as MCTs an easily digested and well. Immerse in boiling cupric sulfate solution about 15 min. Boil immersion A 15 min.
Zinc loses electrons and it is oxidized while copper II ions gain electrons and are reduced. The resulting solution will contain colorless zinc ions Zn2aq and copper solid. Definition of a chemical reaction - A process that involves rearrangement of the molecular or ionic structure of a substance as opposed to a change in physical form or a nuclear reaction S5 These are the reactants.
Organocopper chemistry is the science of organocopper compounds describing their physical properties synthesis and reactions. This experiment includes the reaction of zinc with hydrochloric acid. Boil immersion AB 10 min.
Organocopper compounds in organometallic chemistry contain carbon to copper chemical bonds. Magnesium zinc and copper. 0 500 and 1000 mg copper as cupric chloridekg diet were fed in conjuction with 0 5 10 and 20 mg selenium as Na2Se3kg diet.
Tin lid sitting on a tripod or a strip of ceramic. The video includes a discussion of writing balanced equations for all the observed. The toxicity of silver was significantly less in the zinc-pretreated cells.
Answer 1 of 25. We can conveniently express these two processes by the following two half-reactions which add to give the overall redox reaction. This reagent is widely used in the selective flotation of leadcopperzinc and copperzinc ores to depress sphalerite pyrite and certain copper sulfides.
This occurs at concentrations of about 2 mg Zn 2 L. The shining silver crystals are visible on the Copper wire. CuSO4 solution has a blue colour while ZnSO4 solu.
Laction de lamidure de sodium de baryum ou de potassium sur la pyridine conduit à la 2-aminopyridine. The reaction of R--2-bromooctane a secondary alkyl halide.
Illustrated Glossary Of Organic Chemistry Methoxide
Without exposure to air the crude ketone is immediately slurried with a little ice water and filtered.

Potassium methoxide reaction. In 1900 Jackson and Gazzolo reacted trinitroanisole with sodium methoxide and proposed a quinoid structure for the reaction product. S N 2. First tert-butoxide is a poorer nucleophile than smaller alkoxides like ethoxide methoxide and so on in nucleophilic substitution reactions like the S N 2.
So what does this mean for the chemical reactions of t-butoxide. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products. HA_aqH_2O_l rightleftharpoons H_3O_aqA_aq label1651 The equilibrium.
The base can be used in an amount of 30 to 60 equivalents preferably 40 to 45 equivalents relative to compound F. The reaction mix is kept just above the boiling point of the alcohol around 160 F to speed up the reaction and the reaction takes place. It has many industrial and niche applications most of which exploit its caustic nature and its reactivity toward acids.
Keyword sigma aldrich Showing 1-30 of 553 results for sigma aldrich within Products. Excess alcohol is normally used to ensure total conversion of the fat or oil to its esters. We can understand the acid-catalyzed reaction by considering the resonance forms shown below.
Either form can be decomposed by adding enough dry hydrocarbon solvent eg heptane to reduce the hydride concentration below 5 and then adding excess t-butyl alcohol drop wise under nitrogen with. Along with sodium hydroxide NaOH KOH is a prototypical strong base. In 1895 Cornelis Adriaan Lobry van Troostenburg de Bruyn investigated a red substance formed in the reaction of trinitrobenzene with potassium hydroxide in methanol.
The reaction of alkali cellulose with methyl chloride is under strictly controlled conditions. In 1902 Jakob Meisenheimer observed that by acidifying their reaction product the starting. Empirical Formula Hill Notation.
143 The barium and ammonium salts were also prepared via this method but showed inferior stability to the potassium salt which was described as being thermally stable and non. Care must be taken to monitor the. Hydrogen chloride solution Hydrochloric acid solution.
Cool to -25 and the product is filtered and washed while still in the funnel with cold ethanol and ether. La réaction peut avoir lieu à sec mais en général elle seffectue dans les solvants aromatiques en ébullition. The name of the reaction was coined after Alexander William Williamson developed it in 1850.
For example the general equation for the ionization of a weak acid in water where HA is the parent acid and A is its conjugate base is as follows. KOH is noteworthy as the precursor. Potassium p-toluenephosphonate 433-860-4 015-196-00-3 reaction mass of.
Resonance stabilized View Answer. Sodium methoxide is added 17 g and the mixture is stirred for 10 min at -10 to -12. Williamson Ether Synthesis is a reaction that uses deprotonated alcohol and an organohalide to form an ether.
Under acid-catalyzed conditions the epoxide oxygen is protonated and the ring opens in the opposite direction when the epoxide reacts with methanol. Potassium hydroxide is an inorganic compound with the formula K OH and is commonly called caustic potash. And the methoxide base is regenerated.
The kinds of bonding in sodium methoxide NaOCH_3 isare A. An estimated 700000 to 800000 tonnes were produced in 2005. Being designer solvents they can be modulated to suit the reaction conditions therefore earning the name task specific ionic liquids Though primarily used as solvents they are now finding applications in various fields like catalysis electrochemistry spectroscopy and material.
Potassium and sodium hydride KH NaH in the dry state are pyrophoric but they can be purchased as a relatively safe dispersion in mineral oil. Examples of the base include inorganic bases such as sodium hydroxide potassium hydroxide lithium hydroxide and sodium hydride and metal alkoxides such as sodium methoxide and potassium tert-butoxide preferably lithium hydroxide and sodium And methoxide. A mixture of ionic and covalent E.
Methyl ethyl 2-hydroxymethylcarbamoylethylphosphonate 435-960-3 Carc. Analytical 4 ACS reagent 2 BioReagent 2 Technique. The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases.
The first reports on potassium organotrifluoroborates appeared in the literature in 1960 when Chambers prepared CF 3 BF 3 K by treatment of Me 3 SnCF 3 with BF 3 gas followed by a work-up with aqueous KF. Wash with ice water ethanol then ether all cold to yield 16 g of product melting at 145-147. Potassium tert-Butoxide Is A Poorer Nucleophile Than Other Alkoxides Due To Steric Hindrance.
Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. Recommended reaction time varies from 1 to 8 hours and some systems recommend the reaction take place at room temperature. In this reaction step the hydroxyl groups -OH on the anhydroglucose monomers of the cellulose chain are partially replaced by methoxide groups -OCH3 after etherification.
Dans cette réaction les. Williamson Ether Synthesis usually takes place as an S N 2 reaction of a primary alkyl halide with an alkoxide ion. The structure of ethers was proved due to this chemical reaction.
Les groupes hydroxyle sulfate ou amide en position 2 ou 6 sur la pyridine peuvent aussi être substitués au cours de cette réaction 26. Decomposition of potassium or sodium hydride. Sort by Relevance.
Ionic liquids have emerged as an environmentally friendly alternative to the volatile organic solvents. The positive charge is more stable on the.
Heparinized rat blood containing sodium 35S-sulfide was perfused through isolated rat lungs kidney or liver. 2001 No CDTech A New Process for.

Draw The Mechanism For The Following Reaction With Nash Study Com
Unlike Grignard reagents alkyl lithiums can be prepared and used in a variety of hydrocarbon solvents hexane pentane etc.
Sodium hydrosulfide reaction. Only small amounts of 35S-sulfate were detectable possibly due to the absence of sulfide oxidase. 07272021 On July 27 2021 a release of 100000 pounds of acetic acid during a maintenance event at the LyondellBasell facility in La Porte TX resulted in fatal injuries to two contra. 1000 454 Sodium hypochlorite.
5000 2270 Sodium hydroxide. Among the inorganic depressants lime is the cheapest and best for iron sulphides while zinc sulphate sodium cyanide and. It is a soft silvery-white highly reactive metal.
Aghorn Operating Waterflood Station Hydrogen Sulfide Release. Sort by Relevance. Among organic depressants starch and glue find widest application.
If added in sufficient quantity starch will often depress all the minerals present in an ore pulp. Relative reaction rates for different morphological forms of pyrite are described in the order of marcasite framboidal pyrite. The rate and extent of sulfide oxidation varied from one organ to another.
Keyword sigma aldrich Showing 1-30 of 553 results for sigma aldrich within Products. C 2 H 5 SO 2 Cl chlorides of sulfonic acids by heating the salts of esters of sulfuric acid with potassium hydrosulfide and by heating the alcohols with phosphorus pentasulfide. 5000 2270 Sodium.
Sodium is an alkali metal being in group 1 of the periodic table. The Electronic Code of Federal Regulations eCFR is a continuously updated online version of the CFR. Empirical Formula Hill Notation.
MIBC was used as the frother. The common ion effect may be used to obtain almost complete removal of these gases by aeration. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
Sodium is a chemical element with the symbol Na from Latin natrium and atomic number 11. Analytical 4 ACS reagent 2 BioReagent 2 Technique. Administration of sodium hydrosulfide an alkali salt of hydrogen sulfide at doses of 10 and 30 mgkg corresponding to sublethal and lethal doses 066 and 20 LD50 resulted in significant increases in regional catecholamine levels of the rat brain only after the dose of 20 LD50 of sodium hydrosulfide.
If the concentration of one of the ions on the right side of the equation is increased the reaction is driven to the left forming the gas. As the equations above show ionization of the gases in water is a reversible reaction. Photoredox CO 2 fixation reaction.
1000 454 Sodium nitrite. In the isolated perfused lung system 35S-sulfide was oxidized slowly to 35S-thiosulfate. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily.
ModalParticle Mineralogy and Fe. Struct synth landscapedocx Page. KOH c H 2 O l K aq OH aq H 2 O l 5746.
Baypure TM CX Sodium Iminodisuccinate. 05212021 A release of hydrogen sulfide led to the death of one worker as well as a member of the public. Potassium hydroxide dissolving in water to make an infinitely dilute solution.
This reaction is versatile. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More.
The catalysts used in refinery cracking units are typically solid materials. By the reduction of the sulfo-chlorides eg. Briefly 28 mL sodium citrate 0294 gmL were added into pre-deoxygenated water followed by the blend of 4 mL sodium borohydride 004 gmL and.
Primary secondary and tertiary alkyl halides can be used and also vinyl allyl and aryl halides. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. H 2 O 2 aq H 2 O l ½ O 2 g 9466.
Sodium hydroxide Caustic soda 1310-73-2. 2001 No Burlington Chemical Company Inc. 100 454 Sodium phosphate dibasic.
The ICSC project is a common undertaking between the World Health Organization WHO and. In the case of carbon dioxide and hydrogen sulfide hydrogen ion. Use of a catalyst a material that assists a chemical reaction but does not take part in it in the cracking reaction increases the yield of improved-quality products under much less severe operating conditions than in thermal cracking.
Organolithium Reagents These are also formed via action of the metal with an alkyl halide. Sodium is the sixth most abundant element in the Earths crust and exists. Development of a Practical Model and Process to Systematically Reduce the Environmental Impact of Chemicals Utilized by the Textile and Related Industries abstract Burlington NC.
The free metal does not occur in nature and must be prepared from compounds. The main target users are workers and those responsible for occupational safety and health. 100 454 Sodium methylate.
CaO lime H 2 O l CaOH 2 portlandite 6540. La Porte TX Incident Occurred On. It is not an official legal edition of the CFR.
Typical temperatures are from 850-950 F at much lower pressures of 10-20 psi. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. We subjected an aqueous solution of the sodium salts of HCO 3 1 50 mM and SO 3 2 100 mM at pH 9 to ultraviolet irradiation from Hg lamps with a.
LyondellBasell Fatal Chemical Release. Results and Discussion 31. Reaction of NH 3 and H 2 S to form solid ammonium hydrosulfide.
Lime sodium sulphite cyanide and dichromate are among the best known common depressants. 10149 US_president 41448 Leal_Villa_de_Santiago_de_Managua 185539 Prva_HNL_2007-08 64645 Women_and_Islam 32030 Sara_Cox 55353 Espionage 65210 Thread 11547 Director. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products.
They are colorless liquids which are insoluble in. Sodium sulfide Na 2 S and sodium hydrosulfide NaHS were tested for surface cleaning of the tarnished minerals and framboidal pyrite particularly for Ore B. The mercaptans may be prepared by the action of the alkyl halides on an alcoholic solution of potassium hydrosulfide.
Hydrogen chloride solution Hydrochloric acid solution. 5000 2270 Sodium phosphate tribasic. Odessa TX Final Report Released On.
Decomposition of aqueous hydrogen peroxide to water. Its only stable isotope is 23 Na. The Code of Federal Regulations CFR is the official legal print publication containing the codification of the general and permanent rules published in the Federal Register by the departments and agencies of the Federal Government.
An Environmentally Friendly and Readily Biodegradable Chelating Agent summary Pittsburgh PA.
Hydrolysis subsequently causes flake formation and flakes can be removed by sand filtration. Stability and structure of carbocations.
Other Oxidation Reduction Reactions
Oxidation occurs when a chemical species loses or gives up electrons to another chemical species.
Iron chloride and tin oxidation reaction. Leaching is a process of 1 Silica 1 Reduction 2 Concentration 3 Refining 4 Oxidation 22. Iron removal from wastewater may be achieved by oxidation of binary iron to tertiary iron. This is done by dipping the iron into molten tin or by electroplating an iron sheet using tinIV chloride as the electrolyte.
IronIII oxide aluminium aluminium oxide iron. Simple S N 2 reaction. Metals in the fourth class are so unreactive they are essentially inert at room temperature.
Once underway the reaction is highly exothermic rapidly reaching temperatures as high as 2000 C well in excess of the melting point of iron 1535 C. When metallic iron oxidation state 0 is placed in a solution of hydrochloric acid ironII chloride is formed with release of hydrogen gas by the reaction Fe 0 2 H Fe 2 H 2. 2 o Benzyl Chloride with HS S N 2 Mechanism.
The cans do not rust as long as the tin coating remains. Valency 1 Valency 2 Valency 3. A redox reaction is the simultaneous occurrence of the two components or half reactions.
The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers electron configurations and chemical properties. Benzyl Chloride with HS S N 2 Reaction. The familiar form white or beta tin and gray or alpha tin which is powdery and of little use.
Redox Reaction is a chemical reaction in which oxidation and reduction occurs simultaneously and the substance which gains electrons is termed as oxidizing agent. To be sure reaction was complete small excess of the SnCl 2 is added and then solution is treated with mercury II chloride. There is enough hydrogen and oxygen in the air to allow the atoms to bond with the iron.
Thus vanadium forms. When iron metal is exposed to air and water usually it turns into rust a. NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reactions Redox Reactions is the eighth chapter in the NCERT Class 11 Chemistry textbookThis chapter is regarded by many as one of the most important chapters in the term I CBSE Class 11 Chemistry Syllabus 2021-22 owing to the fact that the entire field of electrochemistry deals with redox reactions.
After tin plating the inside of the can is coated with a thin layer of plastic. Since the process of oxidation-reduction involves the addition and removal of atoms and charges writing a balanced chemical equation for such reactions is quite a daunting task. S N 2 examples.
Weve all noticed reddish-brown rust on iron nails. Oxidation may be achieved by adding oxygen or other oxidants such as chlorine or potassium permanganate. Redox calculator is an online tool which you can use for free.
The oxidation process provides the electrons necessary for reduction to occur. But thanks to the redox reaction calculator which makes it easier for students and researchers to balance a complicated redox equation in just a second. Possible oxidation states are 23.
S N 1 and S N 2. Refer the following table which gives you oxidation numbers. Electron configuration of Iron is Ar 3d6 4s2.
The practical use of this reaction is to weld railway lines together and could be mentioned. Tin exists in two different forms or allotropes. Allyl Chloride with HS S N 2 Reaction.
It also contains even less active metals such as copper which only dissolves when treated with acids that can oxidize the metal. Ca 2 Iron III. The products of reaction with chlorine usually are chlorides with high oxidation numbers such as iron trichloride FeCl 3 tin tetrachloride SnCl 4 or antimony pentachloride SbCl 5 but it should be noted that the chloride of highest oxidation number of a particular element is frequently in a lower oxidation state than the fluoride with the highest oxidation number.
Reduction occurs when a chemical species receives or gain electrons. Tin is a chemical element with atomic number. Fe 0 Cu 2 Fe 2 Cu 0.
This shows that aluminium is above iron in the reactivity series. Concentration of copper pyrites is done by - 1 Gravity separation 2 Froath floatation process 3 Electromagnetic separation. S N 2 Reaction.
Iron metal is more electropositive than copper and therefore will displace it from its salts. Rust is largely hydrated iron III oxide Fe 2 O 3xH 2 O as a result. During rusting iron combines with oxygen in the air in the presence of water to generate Fe 2 O 3xH 2 O a hydrated iron III oxide.
It is when moisture is added that the oxidation process starts to occur. The reaction rate. 3 Chloride ores 4 None of these as impurity 21.
Iron is present in all wastewaters. This creates the chemical reaction known as oxidation or rust. Table of Common Ions.
The low melting point of tin and its firm adhesion to clean surfaces of iron steel copper and copper alloys facilitate its use as an oxidation-resistant coating material. Tin plating is usually used for making tin cans as tin is very expensive. 2FeCl 3 SnCl 2 2FeCl 2 SnCl 4.
Substrate structure controls substitution mechanism S N 1 or S N 2. The gray form changes to the white above 132 C 558 F rapidly. TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in.
The third class contains metals such as chromium iron tin and lead which react only with strong acids. The tin provides a protective oxide coating to the cans. It is possible to reduce iron 3 using Jones reductor column filled with granules of amalgamated zinc however most commonly reduction is done with tin II chloride.
Iron rusting is an oxidation reaction. This hydrated iron Ill oxide is referred to as rust. Therefore the oxidized species is the reducing agent and.
These metals are ideal for making jewelry or coins because they do. Because the air we breathe has moisture in it oxidation will occur even if there is no water added to the metal. The colour of rust is reddish-brown.
Electron Configuration and Oxidation States of Iron.