There was no correlation between duodenal pH and duodenal soluble Cu concentrations P 098. The product is calcium hydrogen carbonate.
Chemistry Copper Carbonate Acitivity
For ionic compounds with limited solubility in water an equilibrium constant K sp can be defined from the ion concentration in water from the equation.

Copper carbonate solubility. Solubility Product Constants near 25 C. Doi10106313679678 IUPAC Project. K sp M n m A m- n.
It may be determined by direct measure-ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states. Calcium phosphate solubility is 20 mgL and that of calcium fluoride is 16 mgL. The following is for solubility in pure water not with any common ions.
Carbonate mineral any member of a family of minerals that contain the carbonate ion CO 3 2- as the basic structural and compositional unitThe carbonates are among the most widely distributed minerals in the Earths crust. For example in the process of. It is one of the simple methods to purify water.
Alters the release of neurotransmitters. The pH in the small intestine is an important factor in determining solubility of Cu as well as other minerals in the digesta Pang and Applegate 2007. The table below gives calculated values of K.
A tolerance of 1 ppm is established in potable water for residues of copper resulting from the use of the algicides or herbicides basic copper carbonate malachite copper sulfate copper monoethanolamine and copper triethanolamine to control aquatic plants in reservoirs lakes ponds irrigation ditches and other potential sources of potable water. Solubility in hydrochloric acid. Calcium carbonate has a solubility of 14 mgL which is multiplied by a factor five in presence of carbon dioxide.
M m A n s mM n aq nA m-aq. However jejunal pH and jejunal soluble Cu concentration were negatively correlated r 051. If compounds have different solubilities or relative solubilities they can be separated.
The table gives the solubility of potassium nitrate at six different temperatures. Jitka Eysseltováa and Roger Bouaziz Potassium Sulfate in Water JPCRD 2012 41 013103. Dirkse Copper Silver Gold and Zinc Cadmium Mercury Oxides and Hydroxides 1986.
AG105 AG203 and. 7110 9 x2x 2. Thus if K sp Mm An is the equilibrium.
Calcium carbonate 70 Salt 15 DL-Methionine. P block metal carbonate compounds. This compound is rarely encountered because it is difficult to prepare and readily reacts with water moisture from the air.
The chemicals are added to form particles which settle and remove contaminants from water. The solubility of silver carbonate is sensitive to the square of the metal-ion concentration because two silver ions per carbonate ion are necessary to build the solid crystal. Only high-silver content brazing alloys should be used for brazed joints at risk of corrosion eg.
Because of how the solubility constant is defined your answer will be in terms of moles of the compound dissolved per liter of water. A The percentage composition by mass of copper pyrites is Cu 3460 Fe 3052 S 3488 Show by calculation that the empirical formula of copper pyrites is CuFeS 2 3 b Copper is obtained from copper pyrites in a two-stage. Affects cyclic adenosine monophosphate concentrations.
CopperII carbonate or cupric carbonate is a chemical compound with formula CuCO 3. The terms copper carbonate copperII carbonate and. And blocks inositol.
Copper brazing alloys L-ZnCu42 formerly standardized in DIN 813 part 1 and CU301 EN 1044 and the silver solders AG306 and AG304 are preferred for the manganese-containing Cu-Ni alloys. Lithium Carbonate is the carbonate salt of lithium a soft alkali metal with antimanic and hematopoietic activities. Solubility and Related Thermodynamic Quantities of CadmiumII Carbonate in Aqueous Systems JPCRD 2011 40 043104.
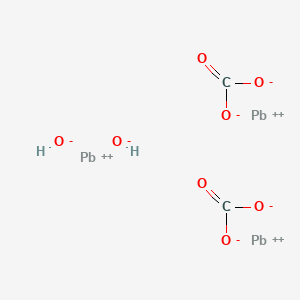
Lead carbonate - PbCO 3. Calcium compounds are more or less water soluble. In this section we are going to discuss solubility and colours of p block metal carbonate compounds.
It is made by reacting copper with chlorine. At ambient temperatures it is an ionic solid a salt consisting of copperII cations Cu 2 and carbonate anions CO 2 3. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
These brazing alloys together with CU305 and AG203 are used for iron-containing alloys. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3. Boiling point - the temperature at which a liquid turns into a gas.
Therefore the form of the solubility product expression is different. SOLUBILITY PRODUCT CONSTANTS The solubility product constant K sp is a useful parameter for calculating the aqueous solubility of sparingly soluble compounds under various conditions. Solubility of calcium and calcium compounds Elementary calcium reacts with water.
Lithium interferes with transmembrane sodium exchange in nerve cells by affecting sodium potassium-stimulated adenosine triphosphatase Na K-ATPase. Solve for x and youll know how soluble the compound is. 7 Copper pyrites is an ore of copper that contains copper iron and sulfur.
It can also be made by reacting copperII hydroxide copperII oxide or copperII carbonate with hydrochloric acid and from pure copper and from 11 solution of hydrogen peroxide and hydrochloric acid where copper first get oxidized to CuO from H2O2 and then reacts with HCl to form CuCl2 reaction goes like this. Ionic Compound Formula K sp. The solubility rules the technique ie when the product of ion concentrations in simple in the solution over the solubility product of the respective solid the precipitation occurs.
Basic copper carbonate is a chemical compound more properly called copperII carbonate hydroxideIt is an ionic compound a salt consisting of the ions copperII Cu 2 carbonate CO 2 3 and hydroxide OH. Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution. Melting point - the temperature at which a solid turns into a liquid.
The name most commonly refers to the compound with formula Cu 2 CO 3 OH 2It is a green crystalline solid that occurs in nature as the mineral malachite. Aluminium carbonate is not stable in the water and hydrolysis to aluminium hydroxide white precipitate in the water. Aluminium carbonate - Al 2 CO 3 2.
You may need a calculator to find the final answer. The positive identification of carbonate minerals is aided greatly by the fact that the carbon-oxygen bond of the CO 3 group in carbonates becomes unstable and breaks down in the presence of hydrogen ions H available in acidsThis is expressed by the reaction 2H CO 2 3 H 2 O CO 2 which is the basis for the so-called fizz test with dilute. The treated water is reused whereas the settled portion is.
The crystal structure of many carbonate minerals reflects the trigonal symmetry of the carbonate ion which is composed of a carbon atom centrally located in an. Where M m A n is the slightly soluble substance and M n and A m-are the ions produced in solution by dissosiation of M m A n. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
Co 3 AsO 4 2. Lithium nickel manganese cobalt oxides abbreviated Li-NMC LNMC NMC or NCM are mixed metal oxides of lithium nickel manganese and cobaltThey have the general formula LiNi x Mn y Co z O 2The most important representatives have a composition with x y z that is near 1 with a small amount of lithium on the transition metal site.
The influence of preparation conditions on variations in the chemical composition morphology of the biochar powders and degree of graphitization of.

Cobalt oxide vs cobalt carbonate. Zirconium Chloride ZrCl. However their commercial applications have been limited due to lower energy density and higher cost. Review the chemical compatibility of Teflon and PTFE with various chemicals solvents alcohols and other products in the cart below.
Mix the two solutions while still warm and cool to 20. The electrolyte was made up of a certain proportion of cobalt sulphate sodium carbonate potassium hydroxide and water. Webcast on November 2 2021 Energy Fuels Inc.
It has an empirical formula of The template Magnesium is being considered for deletion Mg O and consists of a lattice of Mg 2 ions and O 2 ions held together by ionic bonding. NOT compatible with certain alkali metals and fluorinating agents such as xenon difluoride and cobaltIII fluoride. Zircon ZrSiO 4.
Even though cobalt catalysts do not represent the best solution to carry out GSR reaction Sanchez et al. Since there are two bromines each with a charge of -1. Co 3 PO 4 2.
CoCr 2 O 7. However it is usually added in the mineral mix at approximately 10 ppm to ensure no deficiencies. Most of the rock is unwanted material typically referred to as gangue.
The primary deficiency symptom is loss of appetite and poor growth. Similar to chlorine bromine ceBr is also a halogen with an oxidation charge of -1 ceBr-. Li Li and enabled a 40 V.
Cobalt II Hydrogen Phosphate. Zeolites X. Supercapacitors also known as electrochemical capacitors are kind of promising energy storage devices because of their high power density long life and high rate capability.
This work expansively reviews the recent developments of. Garnet Y 3 Al 5 O 12. Copper-containing rock or copper ore holds only a small percentage of copper.
NdPr Rare Earth Oxide China US123977t vs US122964t. Scandium Chloride Sc 7 Cl 10. CobaltUranyl Acetate TS Dissolve with warming 40 g of uranyl acetate in a mixture of 30 g of glacial acetic acid and sufficient water to make 500 mL.
According to the. Copper I Oxide Cu 2. In commercial NMC samples the composition typically has.
These catalysts favoured the production of H. Kaolinite Al 2 OH 4 Si 2 O 5. A trivalent and pentavalent metalloid element with atomic number 51 that commonly occurs in a brittle metallic silvery white crystalline form and that is used especially in alloys semiconductors and flame-retardant substances see Chemical Elements Table.
Today reported its financial results for the quarter ended September 30 2021. The optimal technical parameters for cobalt binder removal from the PCD layer using the electrolysis technique were determined by changing the cobalt sulphate concentration the. Calcium Carbide CaC 2.
Most forages in the Southeast have adequate levels of cobalt. Vanadium Oxide V 2 O 5. Cobalt LME 3m US59500t vs US59500t.
Hydrofluoric acid cobalt II hydroxide hydroselenic. Dihydroxyaluminium acetylsalicylate 53230-06-1 AlOH 2 C 9 H 7 O 4 Dihydroxyaluminium aspirin. Calcium Carbonate CaCO 3 Polymorphs.
The meaning of antimony is stibnite. Dihydroxy aluminium sodium carbonate 539-68-4 NaAlOH 2 CO 3 2 Carbonato1-Odi-hydroxyaluminium monosodium salt. High-nickel layered oxide cathode materials will be at the forefront to enable longer driving-range electric vehicles at more affordable costs with lithium-based batteries.
Dihydroxyaluminium allentoinate 5579-81-7 AlOH 2 C 4 H 5 N 4 O 4 Aldoxia. Considering metal oxide nanoparticles as important technological materials authors provide a comprehensive review of researches on metal oxide nanoparticles their synthetic strategies and techniques nanoscale physicochemical properties defining specific industrial applications in the various fields of applied nanotechnology. Magnesium oxide Mg O or magnesia is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium see also oxide.
PTFE Chemical Compatibility Chart. In addition we know that ceCoBr2 has an overall neutral charge therefore we can conclude that the cation cobalt ceCo must have an oxidation. Similarly prepare a solution containing 200 g of cobaltous acetate in a mixture of 30 g of glacial acetic acid and sufficient water to make 500 mL.
There are two main types of copper ore of. CoS 2 O 3. Muscovite KAl 2 OH 2 Si 3 AlO 10.
Studied a bimetallic catalyst Ni4 wt -Co4 and 12 wt supported on γ-Al 2 O 3. The Companys quarterly report on Form 10-Q. Aluminium sodium carbonate hydroxide.
Lithium cobalt oxide LiCoO2 2 batteries are made from lithium carbonate and cobalt and feature very stable capacities along with high-specific energy making them a popular choice for use with mobile devices such as smartphones laptops and digital cameras. High-grain diets require more cobalt than forage-based diets and. Lithium carbonate 99 China US27924t vs US27872t.
However in this study the authors evidenced that cobalt acts as a precursor while the catalytic activity is mainly given by Ni. During discharge lithium ions travel from anode. Sodium carbonate potassium hydroxide and water were combined in a mass ratio of 22100.
The comprehensive results regarding the physicochemical properties of carbonaceous materials that are obtained from pistachio shells support their usage as solid fuels to supply direct carbon solid oxide fuel cells DC-SOFCs. Maintain the temperature at 20. Gallium oxide Dihydrogen monoxide Provide the correct name for the following acids or bases.
Cobalt II Dichromate. In 1979 and 1980 Goodenough reported a lithium cobalt oxide LiCoO 2 11 which can reversibly intake and release Li-ions at potentials higher than 40 V vs. NaOH NH 3 H2SO 3 HCN H2S CaOH 2 H3PO 4 FeOH 3 Write the correct chemical formula for the following acids or bases.
We would like to show you a description here but the site wont allow us. Magnesium hydroxide forms in the presence of water. Cobalt functions as a component of vitamin B-12 which is synthesized in the rumen by bacteria.
Determine the oxidation state of cobalt in ceCoBr2. A continued push to. Internally they are composed of a cobalt oxide cathode and a carbon graphite anode.
Molybdenum Disulfide MoS 2. Polytetrafluoroethylene is very non-reactive and ideal for use with most chemicals. Co 3 N 2.
Complete these in lab and on your own time for practice. LiCIO3 lithium chlorate 10.

How To Write The Formula For Lead Ii Carbonate Youtube
CH 3 COO C 2 H 3 O 2.
Lead carbonate formula. Lithium Carbonate USP is a white crystalline powder with molecular formula Li 2 CO 3 and molecular weight 7389 gmol. It is a strong base and acts as an antacid. Ammonium cyanide NH4CN 5.
Cadmium phosphate Cd3PO42 9. The chemical formula calculator also contains the names of a range of. The mechanism of action of Lithium as a mood stabilizing.
This inorganic compound is water-soluble and when dissolved in water it forms carbonic acid and sodium hydroxide. This way students can see that the ions combine in whole number ratios in order to produce a neutral chemical species. 1 NaF is sodium fluoride 2 K2CO 3 is potassium carbonate 3 MgCl 2 is magnesium chloride 4 BeOH 2 is beryllium hydroxide 5 SrS is strontium sulfide 6 Cu 2S is copper I sulfide 7 ZnI 2 is zinc iodide 8 Ca 3PO 42 is calcium phosphate 9 NH 4I is ammonium iodide 10 MnNO 33 is manganese III nitrate.
Chemical Formula 11 calcium bromate 12 carbon monoxide 13 potassium oxide 14 antimony tribromide 15 zinc phosphate 16 copper II bicarbonate 17 dinitrogen tetroxide 18 manganese IV carbonate 19 lead IV nitride 20 pentacarbon decahydride. It is also known as Soda crystals soda ash washing soda. Sodium sulfate Na 2 SO 4.
It is an essential raw material in glass chemicals detergents and other important industrial products. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. Potassium nitrate KNO3 11.
E-mail to a friend. White lead is the basic lead carbonate 2PbCO 3 PbOH 2. NiS nickel II sulfide Write the chemical formula for each of the following.
Potassium carbonate K 2 CO 3 2. The empirical formula for Lithium Citrate is C 6 H 5 Li 3 O 7. Use the stock form for the transition metals.
CO 3 4 Pb. This method will also work with lead carbonate or lead oxide. Lead carbonate - PbCO 3.
PO 4 3. Lithium - Clinical Pharmacology Mechanism of Action. MgOH2 magnesium hydroxide 9.
C 2 H 3 O 2 2 Pb. Sodium carbonate is a diazonium salt of carbonic acid with chemical formula Na 2 CO 3. The mechanism of action of lithium as a mood stabilizing agent is unknown.
Some Metallic Cations. 987 989 respectively of total lead admin was fecally excreted. It was formerly used as an ingredient for lead paint and a cosmetic called Venetian ceruse because of its opacity and the satiny smooth mixture it made.
Sodium carbonate Na2CO3 6. Sodium thiosulfate ____Na2S2O3_____ 3. BaSO4 barium sulfate 2.
C 2 H 3 O 2 4 Ld. How to identify solubility of metal carbonate. Section B Write the formula of the ionic compounds containing polyatomic ions 1.
CaCO3 calcium carbonate 11. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
Ratio of urinary lead excretion to total lead admin was very small 024 009 respectively. You should complete this by Sunday. Bicarbonate HCO 3.
NH42SO4 ammonium sulfate 20. Copy this to my account. Optical constants of CaCO 3 Calcium carbonate Calcite Ghosh 1999.
What is the correct formula unit for the ionic compound leadII chromate. Zinc phosphate Zn3PO42 4. It was not until just a few years ago when the manufacturer removed lead acetate from the hair coloring product and as of July 2018 the ingredients in Grecian Formula are water isopropyl alcohol triethanolamine bismuth citrate sodium thiosulfate fragrance and panthenol.
Lithium carbonate USP is a white light alkaline powder with molecular formula Li 2 CO 3 and molecular weight 7389. Lead II chlorate PbClO32 2. Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box.
Lithium acts as an antimanic. Cr 2 O 7 2. A white precipitate and toxic due to lead ion occurrence.
Potassium carbonate 2K CO 3 2- K 2 CO 3. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Ionic Compounds Naming and Formula Writing.
Names Formula Names Formula ammonium NH4 acetate CH3COO-C2H3O2-bromate BrO3-carbonate CO3 2-chlorate ClO3-chlorite ClO2-chromate CrO4 2-cyanide CN-dichromate Cr2O7 2-hydrogen carbonate bicarbonate HCO3-hydrogen sulfate bisulfate HSO4-hydrogen phosphate biphosphate HPO4 2-hydroxide OH-hypochlorite ClO-iodate IO3-nitrate NO3-nitrite NO2-oxalate C2O4 2-perchlorate ClO4. It is a complex salt containing both carbonate and hydroxide ions. Reaction with Oxygen.
Pb 2 CrO 4. Lead acetate has been. If solubility is like 0005 g100 ml water solubility is very low and deposit as a precipitate in the water.
PbCl2 lead II chloride 7. Iron II chloride 18. Reaction of Group 1 Elements.
Zinc iron II iron III gallium silver lead IV chloride ZnCl 2. PbCrO 4 2. Ionic Compound Naming and Formula Writing List 1.
Tin II bicarbonate SnHCO32 10. C 2 H 3 O 2 Pb. Chemical Formula Nomenclature Practice.
Copy this to my account. Copper I sulfite Cu2SO3 8. Chemical formula plays an important role in understanding different concepts of chemistry.
28 zinc carbonate ZnCO3 29 manganese VII arsenide Mn3As7 30 copper II chlorate CuClO32 31 cobalt III chromate Co2CrO43 32 ammonium oxide NH42O 33 potassium hydroxide KOH 34 lead IV sulfate PbSO42 35 silver cyanide AgCN 36 vanadium. B the chemical formula of the compound appears after the arrow. What is the correct formula unit for the ionic compound leadIV acetate.
Na2S sodium sulfide 17. In 1998 in terms of. Soda ash is the trade name for sodium carbonate a chemical refined from the mineral trona or sodium-carbonate-bearing brines both referred to as natural soda ash or manufactured from one of several chemical processes referred to as synthetic soda ash.
Sulfur dioxide ____SO2_____ 2. The names are found by finding the intersection between the cations and anions. Give the formula for the following.
Adult male dogs were given daily doses of 341 mg and 682 mg basic lead carbonate for 60 days. In its pure form it is white powder and odourless. Lithium Carbonate - Clinical Pharmacology Mechanism of Action.
Na2CrO4 sodium chromate 19. White lead occurs naturally as a mineral in which context it is known as hydrocerussite a hydrate of cerussite. KNO3 potassium nitrate 8.
Lead IV dichromate PbCr2O72 7. Ionic Compound Formula K sp. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3.
When solubility of metal carbonate is given for 100 ml you can see solubility is low or high. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another. ONE-SCHOOLNET Periodic Table.
Strontium acetate SrC2H3O22 3. The entire group 1 metal can react with oxygen to form metal oxide. Examples of understanding solubility from solubility value.
Formula to name problems. Chemical formulas can be. E-mail to a friend.
OH2 barium hydroxide 16. Solubility Product Constants near 25 C. C 2 H 3 O 2 4 Pb.
Atomic Mass of Atoms. Density of Potassium carbonate.

What Is The Molecular Formula And Molar Mass Of Potassium Bicarbonate Youtube
Computing Molecular Mass for a Covalent Compound Ibuprofen.

Molecular mass of potassium carbonate. 105033 View Pricing Availability. We can often identify molecular compounds on the basis of their physical properties. 56580 Similar chemical formulas.
Potassium is an essential constituent for plant growth and is found in most soils. Gas chromatography-mass spectrometry data were collected using an Agilent Technologies GC-MS instrument consisting of a 7890B GC inlet 5977B mass spectrometer electron impact ionization EI and. K 2 CO 3.
What is the estimated average requirement of potassium in moles. CO3- because the background. These molecular compounds covalent compounds result when atoms share rather than transfer gain or lose electrons.
Potassium permanganate is an inorganic compound with the chemical formula KMnO 4 and composed of K and MnO 4It is a purplish-black crystalline salt that dissolves in water to give intensely pink or purple solutions. What is the molar mass of sodium carbonate Na2CO3. Covalent bonding is an important and extensive concept in chemistry and it will be treated in considerable detail in a later chapter of this text.
Under normal conditions molecular. An alloy of sodium and potassium NaK is used as a heat-transfer medium. Calculate the molecular or formula mass of each of the following.
K 2 CrO 4. You can also share. MgC 2 H 3 O 2 2.
The same goes for potassium tartrate at 1 g and for potassium cyanide at only 50 mg. 3909832 120107 1599943 Percent composition by element. This compound is also known as Potassium Carbonate.
H 3 x 1. Boiling Point of Potassium carbonate. Potassium carbonate K2CO3 is a white salt soluble in water insoluble in ethanol which forms a strongly alkaline solutionIt can be made as the product of potassium hydroxides absorbent reaction with carbon dioxideIt presents a large capacity to absorb moisture.
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Referring to the periodic table the atomic mass of K is 3910 amu and so its molar mass is 3910 gmol. Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and.
00 amu 3 The molecular mass of H2SO4 98. It is used as gunpowder in explosives such as. If molecular formula calculator add up the total value which is 12 46 48 106.
Molecular Weight Molar Mass. Potassium phosphate NH 4 2 SO 4. Melting Point of Potassium carbonate.
5611 gmol Chemical Formula. Cr 3 PO 4 2. PROBLEM PageIndex4 Determine the molecular mass.
Potash caustic CAS. Potassium dichromate is lethal at between 6 and. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
Download Product Safety Card. Molecular weightmolar mass of K 2 CO 3. Sodium hydrogen carbonate or sodium bicarbonate.
Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. The tool is designed so you can flip between different parts of a problem set. The molar mass distribution or molecular weight distribution describes the relationship between the number of moles of each polymer species N i and the molar mass M i of that species.
Potassium carbonate is lethal to adults at doses above 15 g. Molar Mass of Potassium permanganate KMnO4 Molar Mass of Sodium carbonate Na2CO3 Molar Mass of Sodium nitrate NaNO3 Molar Mass of Sulfurous acid H2SO3 Molar Mass of Zinc sulfate ZnSO4 Bookmarking Save and Share Results. IronIII hydroxide or ferrous hydroxide.
Convert grams K2CO3 to moles or moles K2CO3 to grams. Solution The mass of K is provided and the corresponding amount of K in moles is requested. In linear polymers the individual polymer chains rarely have exactly the same degree of polymerization and molar mass and there is always a distribution around an average value.
Click here to see a video of the solution. Potassium Nitrate structure KNO 3 Structure Potassium Nitrate Structure. The given mass of K 47 g is a bit more than one-tenth the molar.
Sodium bicarbonate baking soda NaHCO3 can be purified by dissolving it in hot water 60 C filtering toexample. Acids are compounds in which the cation is. In the NaOH.
Potassium hydroxide MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents. Therefore the molar mass of Na2CO3 is 106 gmol. Many potassium salts are of utmost importance including the hydroxide nitrate carbonate chloride chlorate.
A P 4 b H 2 O c CaNO 3 2 d CH 3 CO 2 H acetic acid e C 12 H 22 O 11 sucrose cane sugar. 16 x 3 48. Since sodium carbonate contains one carbon atom two sodium atoms and three oxygen atoms the molecular weight is.
Most potassium 95 goes into fertilizers and the rest goes mainly into making potassium hydroxide KOH by the electrolysis of potassium chloride solution and then converting this to potassium carbonate K 2 CO 3. Substance MW or FW molar mass Fe 55. Potassium Nitrate KNO 3 Uses.
120 x 1 12. It is used as a form of fertilizer as it contains all the macronutrients needed for the plants to grow. Acids and Acid Salts.
We recommend you bookmark it so you can refer back to it. Potassium alum may cause stomach complaints and nausea at concentrations as low as 2 g and may be corrosive and even lethal in higher concentrations. 230 x 2 46.
Potassium carbonate goes into glass manufacture expecially the glass used to make televisions while potassium hydroxide is used to make liquid soaps and detergents.
For this type of reaction to occur the reduction potential of the reactant receiving the electrons must be lower than the reduction potential of the reactant giving up electrons. Cadmium hydroxide CdOH 2 2510 14.

How To Write The Formula For Copper Ii Bicarbonate Youtube
The copper metal reacts with oxygen resulting in the formation of an outer layer of.
Copper hydrogen carbonate. MercuryI Hg 2 2. Copper is a chemical element with the symbol Cu from Latin. CaSO4 calcium sulfate 5.
Ionic bonds are atomic bonds created by the attraction of two differently charged ionsThe bond is typically between a metal and a non-metal. Calcium hydroxide CaOH 2 5. Cuprum and atomic number 29.
Copper will not react with hydrochloric acid. Hydrogen storage requires the reduction of an enormous volume of gas and an objective in hydrogen storage is to pack hydrogen as close as possible ie. FeC2H3O22 iron II acetate 4.
And blocks inositol. Answer 1 of 25. FeNO22 iron II nitrite 9.
Cu 2 OH 2. SnSO4 tin II sulfate 14. Cu2CO3 copper I carbonate 8.
At the same time fluctuations in the levels of iron and zinc which are essential elements were determined in the tissuesLevels of copper iron and zinc in blood were. Calcium hydrogen phosphate CaHPO 4 110 7. Hydroxide ions from say sodium hydroxide solution remove hydrogen ions from the water ligands attached to the copper ion.
Cu 2 OH. Cadmium sulfide CdS 810 28. If the atmosphere consists of high humidity moisture then this process is faster.
Water Hydrogen sulfate Hydrogen phosphate Hydrogen nitrate. This is our newest publication and has been created to support the school technician profession in Scotland. BaClO42 barium perchlorate 11.
Rats were fed a diet contain basic cupric carbonate at doses of 0 70 220 670 and 2000 ppm as cupric hydroxide and 12 months the levels of copper in the blood and tissues were determined by atomic absorption analysis. NO 2 Potassium. Ionic bonds also melt at high temperatures.
As a mineral it is known as tenoriteIt is a product of copper mining and the precursor to many other copper-containing products and chemical compounds. 1 Cs ion and 1 HCO 3-ion. Cadmium oxalate CdC 2 O 4 1510 8.
K2Cr2O7 potassium dichromate 6. Oxidation is a phenomenon whereby an element loses electrons andor hydrogen on interacting with another element. Copper sulphate blue stone blue vitriol are all common names for pentahydrated cupric sulphate CuSO 4 5H 2 O which is the best known and the most widely used of the copper salts.
ZnHCO32 zinc bicarbonate 15. Carbonate Iron II hydroxide Iron II sulfate Iron II phosphate Iron II nitrate Mg2Magnesium chloride Magnesium carbonate Magnesium hydroxide Magnesium sulfate Magnesium phosphate Magnesium nitrate HHydrogen chloride Hydrogen carbonate. CdOH2 cadmium hydroxide 13.
Indeed it is often the starting raw material for. The chemical element Calcium Ca atomic number 20 is the fifth element and the third most abundant metal in the earths crust. Calcium chromate CaCrO 4 7110 4.
It is a soft malleable and ductile metal with very high thermal and electrical conductivityA freshly exposed surface of pure copper has a pinkish-orange colorCopper is used as a conductor of heat and electricity as a building material and as a constituent of various metal alloys such as sterling. The formula unit for the ionic compound cesium hydrogen carbonate consists of which of the following. 2 Cs ions and 1 CO 3 2-ion.
Lithium Carbonate is the carbonate salt of lithium a soft alkali metal with antimanic and hematopoietic activities. What is the correct formula unit for the ionic compound copperII hydroxide. AgNO3 silver nitrate 10.
Uses of Copper Sulphate. To increase the density of hydrogen either work must be applied to compress the gas or the temperature must be decreased below the critical. H 3 O LeadII Pb 2.
H 3 O hydronium. Lithium interferes with transmembrane sodium exchange in nerve cells by affecting sodium potassium-stimulated adenosine triphosphatase Na K-ATPase. 2 Cs ions and 1 HCO 3 2-ion.
Why and why does this reaction even involve electron. Copper oxide is a base. Optical constants of Cu Copper Johnson and Christy 1972.
Calcium fluoride CaF 2 5310 9. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. 1 Cs 2 ion and 2 HCO 3-ions.
Alters the release of neurotransmitters. CopperI Cu 2 copperII Polyatomic positive ions often have common names ending with the suffix -onium. A black solid it is one of the two stable oxides of copper the other being Cu 2 O or copperI oxide cuprous oxide.
Carbonate CO3 2-chlorate ClO3-chlorite ClO2-chromate CrO4 2-cyanide CN-dichromate Cr2O7 2-hydrogen carbonate bicarbonate HCO3-hydrogen sulfate bisulfate HSO4-hydrogen phosphate biphosphate HPO4 2-hydroxide OH-hypochlorite ClO- iodate IO3-nitrate. H aq OH-aq H 2 Ol Pure water is neutral. Calcium carbonate CaCO 3 2810 9.
Once a hydrogen ion has been removed from two of the water molecules you are left with a complex with no charge - a neutral complex. NH 4 ammonium. The metal is trimorphic harder than sodium but softer than aluminiumA well as beryllium and aluminium and unlike the alkaline metals it doesnt cause skin-burns.
It is less chemically reactive than alkaline metals and than the other alkaline-earth. The structure of the bond is rigid strong and often crystalline and solid. 1 Cs 2 ion and 1 CO 3 2-ion.
Similar to iron and aluminum the element copper undergoes the process of oxidation if it is exposed to air. Cu H 2 O 2 CuO H 2 O CuO 2HCl CuCl 2 H 2 O Uses. KHCO3 potassium bicarbonate 7.
Affects cyclic adenosine monophosphate concentrations. Hydrogen carbonate HCO 3 1 sulfite SO 2 2 lithium Li 1 bisulfate hydrogen sulfate HSO 4 1 sulfide S 2 2 nickelII nickelous Ni 2 2 bromate BrO 3 1 thiocyanate SCN 1 potassium K 1 bromide Br 1 thiosulfate S 2 O 3 2 2 A note. In acid-alkali neutralisation reactions hydrogen ions from the acid react with hydroxide ions from the alkali.
Cadmium carbonate CdCO 3 5210 12. TinIV Sn 4. CopperII oxide or cupric oxide is an inorganic compound with the formula CuO.
PbCO32 lead II carbonate 12. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. Negative ions that consist of a single atom are named by adding the suffix -ide to the stem of the name of the element.
To reach the highest volumetric density using as little additional material and energy as possible. Cu2CO3 copper I carbonate 3. It can also be made by reacting copperII hydroxide copperII oxide or copperII carbonate with hydrochloric acid and from pure copper and from 11 solution of hydrogen peroxide and hydrochloric acid where copper first get oxidized to CuO from H2O2 and then reacts with HCl to form CuCl2 reaction goes like this.
