C OOH which is acetic acid. The ester which is organic compound derived from a carboxylic acid and an alcohol in which the OH of the acid is replaced by an OR group looks somewhat like an ether and also somewhat like a carboxylic acid.

Methyl Butanoate Functional Group Covalent Bonding Carbon Molecule
Theres generally no need to come up with general formulas for things in organic chemistry because all of the structural elements can be combined in multiple ways and they are still carboxylic acids.

Carboxylic acid general formula. In this carboxyl group there exists a carbon which shares a double bond with an oxygen atom and a. Its general molecular formula is. A carboxylic acid has the formula CH_3COOH that is a central carbon atom with an oxygen atom double bonded to it at the top an OH group bonded to it at a 135 degree angle and an R group which in this case is a methyl group with the formula CH_3 bonded to the central carbon atom at a 225 degree angle.
When an acid is dissolved in water it ionizes as-. Remember that the O atoms are not joined to each. Carboxylic acids are organic acids that are composed of a side chain attached to the carboxyl group eq-COOH eq.
The accepted general formula for a carboxylic acid is RCOOH where R can be anything. Esters are represented by the formula RCOOR where R and R are hydrocarbon groups. Carboxylic acids take their names from their parent alkanes.
For example ethane is the parent alkane of ethanoic acid. General formula of acids is. Carboxylic acids are the most common type of organic acid.
You can the have di-carboxylic acids. The general molecular formula for carboxylic acid is C n H 2n1 COOH. Ethanoic acid has the formula CH 3 COOH and this structure.
In general dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acidsDicarboxylic acids are also used in the preparation of copolymers. Carboxylic acids are weak acids which react in the same way as dilute mineral acids. General formula The general formula for the carboxylic acids is C n H 2n1 COOH where n is the number of carbon atoms in the molecule minus 1.
The general molecular formula for carboxylic acids is C n H 2n1 COOH. Answer 1 of 3. The general formula of a carboxylic acid is R-COOH were COOH refers to the carboxyl group and R refers to the rest of the molecule to which this group is attached.
There are five different compounds with this general formula where the only. A carboxylic acids general formula is R-COOH where COOH denotes the carboxyl group and R denotes the rest of the molecule to which this group is attached. Carboxylic acid any of a class of organic compoundsin which a carbonC atom is bonded to an oxygenO atom by a double bond and to a hydroxyl group OH by a single bond.
Carboxylic acids contain at least one carboxyl group. A carboxylic acid group always lies at the end of a carbon chain. Definition A carbon oxoacid acid carrying at least one COOH group and having the structure RCOOH where R is any any monovalent functional group.
There is carbon in this carboxyl group that has a double connection with an oxygen atom and a single bond with a hydroxyl group. This is what we call trying too hard. K e q R C O O H 3 O H 2 O R C O O H K a K e q H 2 O R C O O H 3 O R C O O H Where K e q is the equilibrium constant and K a is the ionization constant of the acid or dissociation constant of the acid.
The accepted general formula for. For acetic acid it is CH3. For formic acid it is H.
Carboxylic acids are organic molecules which form an homologous series with the general formula C n H 2n O 2. For n1 we get C H 3. In general carboxylic acids are represented by the formula RCOOH where R is a hydrocarbon group.
What is a Carboxylic Acid. The general molecular formula of the carboxylic acid homologous series is rmC_rmnrmH_2rmnrmO_2 where rmn 123 ldots. They are named like hydrocarbons according to the number of carbon atoms in the molecule.
A dicarboxylic acid is an organic compound containing two carboxyl functional groups COOH. A carboxylic acids general formula is R-COOH where the COOH formula denotes the carboxyl group and R denotes the rest of the molecule to which this group is linked. Name of Carboxylic Acid.
Oxalic acid for example is a carboxylic acid where R is also a carboxylic acid. A fourth bond links the carbon atom to a hydrogenH atom or to some other univalent combining group. There is carbon in this carboxyl group that has a double bond with an oxygen atom and a single bond with a hydroxyl group.
The general molecular formula for dicarboxylic acids can be written as HO 2 CRCO 2 H where R can be aliphatic or aromatic. Carboxylic acids are nothing but organic compounds in which the carbon atom is. Carboxylic acids with two or more carboxyl groups attached are called.
In the IUPAC nomenclature of these compounds the suffix. HCOOH - methanoic acid Formic acid CH 3 COOH - ethanoic acid acetic acid.

Organic Chemistry Notes Full Course Pdf Notes Chemistrynotes Com Organic Chemistry Cheat Sheet Organic Chemistry Notes Chemistry Notes
The most effective pH buffering range for a buffer that have ka value 66104 A 560 - 760 318 - 418 660 - 860 218 - 418 470.

Nomenclature of carboxylic acids. Carbon compounds containing a carboxyl functional group COOH are called carboxylic acids. Weve got you covered with our online study tools. The tart flavor of sour-tasting foods is often caused by the presence of carboxylic acids.
Experts answer in as little as 30 minutes. It provides the general formula for carboxylic ac. CH3CH2CH2COOH Butyric acidButanoic acid CH3 2CHCOOHIsobutyric acid 2-Methylpropanoic acid.
Generally these organic compounds are referred to by their trivial names which contain the suffix -ic acid. QA related to Nomenclature Of Carboxylic Acids. In a carboxyl group a carbon atom is bonded to an oxygen atom by a double bond left rmC.
Count the longest carbon chain that contains the carboxylic acid and use the base name from the table here. Nomenclature of Carboxylic acids. Nomenclature of carboxylic acids Since carboxylic acids are amongst the earliest organic compounds to be isolated from nature a large number of them are known by their common names.
Complete your research with top quality products. Thus the name alkanoic acid. There are of following rules for naming the nomenclature of carboxylic acid.
Salts are named as ionic salts ie. CH3COOH Acetic acid Ethanoic acid. CH3CH2COOH Propionic acid Propanoic acid.
For example the name formic acid was given since it was first obtained by the distillation of red ants Latin. This organic chemistry video tutorial shows you how to name carboxylic acids including IUPAC Nomenclature. Some examples describing the nomenclature of carboxylic acids as per IUPAC guidelines are provided below.
An example of a trivial name for a carboxylic acid is acetic acid CH 3 COOH. When such carboxylic acids are substituted the carbon of the. Physical Properties Carboxylic Acids - Nomenclature and Properties 7.
Ad Enabling you to solve the toughest problems in life science. Carboxylic Acids Carboxylic acids are weak organic acids which contain the carboxyl group RCO2H. The general structural formula of the carboxylic acid is rmRCOOH where rmR stands for any side group comprising rmH or an alkyl group or another rmC bonded to a certain chain.
Nomenclature of carboxylic acid. Structure Common name IUPAC name. Change the ending of the parent name.
Acidity of Carboxylic Acids The H of COOH is acidic and will readily react with bases to give carboxylate salts. The longest continuous carbon chain must contain the carboxylic group which gets the lowest number. Need more help understanding nomenclature of carboxylic acids.
HCOOH Formic acidMethanoic acid. Systematic Nomenclature of Cyclic Carboxylic Acids 10 The systematic name of a carboxylic acid in which the COOH group is attached directly to a ring is derived by adding a suffix carboxylic acid to the name of the attached cycloalkane or cycloalkene or arene. In the case of molecules containing carboxylic acid and alcohol functional groups the OH is named as a hydroxyl substituent.
Carboxylic acids are named according to following systems. The common names end with the suffix-ic acid and have been derived from Latin or Greek names of their natural sources. The carboxyl group consists of a carbonyl group attached to a hydroxyl group hence its name carboxyl.
Carboxylic Acids - Nomenclature and Properties 6. The naming of carboxylic acids are done by adding suffix oic acid in place of -e of the corresponding alkane. That means when you write the IUPAC name of carboxylic acid you should end the name with oic acid.
This means that the carboxyl group is given the lowest possible location number and the appropriate nomenclature suffix is included. Ad Enabling you to solve the toughest problems in life science. Carboxylic acids are given the highest nomenclature priority by the IUPAC system.
What is the structural formula of the carboxylic acid. Some carboxylic acids with their common names as well as their IUPAC names are given below. Common name of carboxylic acids are given on the basic of the source from which they are obtained.
Iupac Nomenclature of Carboxylic Acids The IUPAC name of a carboxylic acid is derived from that of the longest carbon chain that contains the carboxyl group by dropping the final e from the name of the parent alkane and adding the suffix oic followed by the word acid. RCOH a carboxylic acid O C O O H the carboxyl group C O H O RCOOH RCO2H condensed ways of writing the carboxyl group 3 Nomenclature of Carboxylic Acids 4. Nomenclature of Carboxylic Acids.
Metal first and then anion ending. Complete your research with top quality products. Nomenclature of Carboxylic Acids IUPAC name of Carboxylic Acids.
To name carboxylic acids follow these steps.
4 Spectral Information Expand this section. An organic acid molecule consisting of a chain of carbon molecules and a carboxylic acid -COOH group.
9 8 Carboxylic Acids And Esters Chemistry Libretexts
They are components of many foods medicines and household products.

Foods that contain carboxylic acids. Fatty acids are long-chain carboxylic acids that may contain one or more carboncarbon double bonds. Not surprisingly many of them are best known by common names based on Latin and Greek words that describe their source. Our objective was to develop a comprehensive food database consisting of the total antioxidant content of typical foods as well as other dietary items such as traditional medicine plants herbs and.
Oxalic acid is present in tomatoes spinach and especially in carambola and rhubarb. This difference in acidity can be exploited to separate carboxylic acids and phenols from each other in an organic layer. Rhubarb leaves and unripe carambolas are toxic because of high concentrations of oxalic.
While many phenols dissolve poorly in water 83 g100 mL at 20. It has a role as a metabolite and a reagent. Its structure was established by Johannes Wislicenus in 1873.
1 Structures Expand this section. Why are carboxylic acids acidic. Acetic anhydride is an acyclic carboxylic anhydride derived from acetic acid.
Molecules with the same functional group tend. Knowing that this monounsaturated fat comes with a host of health benefits you may be wondering what foods are high in oleic acid. What foods contain carboxylic acids.
Too much fat in the diet can contribute to obesity. Carboxylic acids are weak organic acids which contain the carboxyl group RCO2H. The nitrosated amino acids include N-nitrosoproline N-nitrososarcosine N-nitrosothiazolidine-4-carboxylic acid N-nitrosooxazolidine-4-carboxylic acid and N-nitroso-2-methyl-thiazolidine-4-carboxylic acid.
These amino acids are broken down in the muscles instead of the liver helping to enhance energy production and muscle synthesis during. The name reflects the lact-combining form derived from the Latin word lac which means milkIn 1808 Jöns Jacob Berzelius discovered that lactic acid actually L-lactate also is produced in muscles during exertion. Fatty acids are precursors to the molecules that form cell membranes throughout our bodies and.
Humans and other animals need fats and we can convert glucose and other carbohydrates into fats. Natural fruits and vegetables also contain acids. 7 Food Additives.
It is present in citrus fruits and provides them with a sour or tart taste. But fats are not bad. Citric acid is present in oranges lemon and other citrus fruits.
Corn walnut and soybean are rich sources of n-6 polyunsaturated fatty acids while flax seed is fairly unique among plants. Essential amino acids also known as indispensable amino acids are amino acids that humans and other vertebrates cannot synthesize from metabolic intermediates. As you can see most foods contain a mixture of fatty acids.
Branched-chain amino acids cannot be produced by the body and need to be obtained from food sources. It has been hypothesized that plant antioxidants may contribute to the beneficial health effects of dietary plants. The structure of fatty acids varies in any one or more of three ways.
Ester is any of a class of organic compounds that react with water to produce alcohols and organic or inorganic acids. These amino acids must be supplied from an exogenous diet because the human body lacks the metabolic pathways required to synthesize these amino acids12 In nutrition amino acids are classified as either essential or non. Fatty acids can be burned by the body for energy.
With the exception of some specialized fatty acids produced by certain prokaryotes the fatty acid residues in lipids are straight acyl chains with a carboxylic acid group at one end and a methyl group at the other end. Tartaric acid is an important component of some commonly used foods like unripened mangoes and tamarind. 3 Chemical and Physical Properties Expand this section.
5 Related Records Expand this section. Structures of some important nonvolatile N-nitrosamines are shown in Figure 2. However they do react with a strong base like NaOH.
The leucine structure contains an alpha-amino group an alpha-carboxylic acid group and a side chain isobutyl group making it a branched-chain amino acid. Stick margarine is the only product in the figure that contains an appreciable amount of trans fatty acids. Heres a list of the top 20 foods and oils high in oleic acid and the percentage of the acid that makes up the total fat content.
Fatty acids are found in fats oils and as components of a number of essential lipids such as phospholipids and triglycerides. In the study the researchers assessed omega-3 carboxylic acids or omega-3 CA brand name Enova a medication that is derived from fish oil. Esters derived from carboxylic acids are the most common.
The tart flavor of sour-tasting foods is often caused by the presence of carboxylic acids. Swedish chemist Carl Wilhelm Scheele was the first person to isolate lactic acid in 1780 from sour milk. The term ester was introduced in the first half of the 19th century by German chemist Leopold Gmelin.
Dietary plants contain variable chemical families and amounts of antioxidants. What you learn in this chapter about the chemistry of carboxylic. RCOH a carboxylic acid O C O O H the carboxyl group C O H O RCOOH RCO2H condensed ways of writing the carboxyl group.
Carboxylic acids occur widely in nature often combined with alcohols or other functional groups as in fats oils and waxes. 2 Names and Identifiers Expand this section. Femoral neck a portion of the thighbone femur.
Normal carboxylic fatty acid In common usage soap is a commercial cleansing and sudsing agent made of water-soluble salts of fatty and other natural acids Thus by definition soap is differientiated from other deter- gents _3o Basic Characteristics of Detergents The two basic characteristics which distinguish detergents in solution from. Chapter 5 Carboxylic Acids and Esters 3 Nomenclature of Carboxylic Acids 4 Nomenclature of. The STRENGTH trial which began in 2014 encompassed data from 13078 adults at 675 centers in 22 countries.
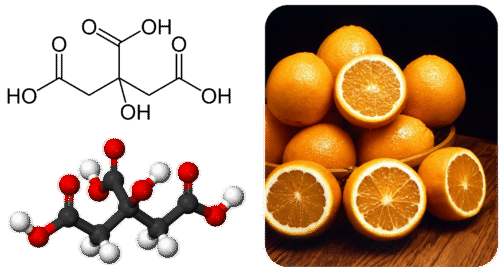
A large carboxylic acid with three ionizable hydrogen atoms is citric acid. There is citric acid in citrus fruits such as oranges and lemons. In plants and animals certain carboxylic acids exist naturally.
Foods and Oils. The carboxylic acids are acidic because of. Foods high in fat are at the top of the food pyramid and these foods have about twice the energy content as carbohydrates.
Number of carbons from the carboxylic acid alpha end to the first carbon in the double bonds. Some other functional groups contain nitrogen atoms such as the amines and amides. Many functional groups contain oxygen atoms such as alcohols ethers aldehydes ketones carboxylic acids and esters.
Most phenols are weak acids pK a 10 and do not react with sodium bicarbonate which is a weak base itself pK a H 2 CO 3637 103.