Copy this to my account. C 25 H 42 N 7 O 17 P 3 S xLi CAS No.

Zinc Sulfide 99 99 Trace Metal Basis 10 Microns Acros Organics Other Inorganic Compounds Chemicals Fisher Scientific
In an experiment a mixture of copper and sulfur was heated to produce a sample of copper sulfide.

Zinc sulfide formula. 3P zinc carbide Zn 2C manganese IV sulfide MnS 2 cobalt II bromide CoBr 2 manganese II carbide Mn 2C phosphorus V nitride P 3N 5 gold I iodide AuI nickel III phosphide NiP iron II bromide FeBr 2 copper II sulfide CuS aluminum sulfide Aℓ 2S 3 silicon iodide SiI 4 lead IV carbide PbC aluminum fluoride AℓF 3 arsenic V nitride As 3N 5 mercury I phosphide Hg 3P cobalt III. 63 Cu 2 S surface exhibits higher adsorption energies than CuS surface which indicates higher role of Cu 2 S or non-stoichiometric Cu x S phases in the adhesion between rubber and brass. Chemical Formulas And Names Of Ionic Compounds.
The obtained ZnIn2S4 catalyst can reduce CO2 to. The iron content is normally less than 25 by weight. NaMnO4 sodium permanganate 50.
It is on the World Health Organizations List of Essential Medicines a list of the most important medication needed in a basic health system. Hg2Cl2 mercury I chloride 57. Chemical Formula Nomenclature Practice.
Both elements exhibit only one normal oxidation state 2 and the Zn2 and Mg2 ions are of similar size. Zinc has many commercial uses as coatings to prevent rust in dry cell batteries and mixed with other metals to make alloys like brass and bronze. 83762 free acid basis Compare Product No.
Use the stock form for the transition metals. E-mail to a friend. 71 silicon dioxide SiO 2 72 nickel III sulfide Ni 2 S 3 73 manganese II phosphate Mn 3 PO 4 2 74 silver acetate AgC 2 H 3 O 2 75 diboron tetrabromide B 2.
Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Although this mineral is usually black because of various impurities the pure material is white and it is widely used as a pigment. You should complete this by Sunday.
Zinc nitrite 70 V 2 S 3 vanadium III sulfide Write the formulas for the following chemical compounds. The chemical formula of sphalerite is ZnFeS. Redness irritation burning sensations dry hair and blistering if the shampoo is left on the skin for too long.
AMPAIRE TALENTS March 22 2020. And 2 deposition from aqueous brine solutions at temperatures in the 300600 C 5721112 F range and at relatively high pressure such as at the seafloor or several kilometres. Zinc iron II iron III gallium silver lead IV chloride ZnCl 2.
63 Zinc sulfide seems to prefer adsorption via sulfur-containing groups whereas preferable adsorption on. NaCIO4 sodium perchlorate 47. Formula writing rules to write the correct chemical formulas for each compound.
Give the formula for the following. Importantly selenium sulfide shampoos have been known to discolor dyed hair and can discolor metallic jewelry so should be removed. Sphalerite often contains trace to minor.
Sphalerite is a sulfide mineral with the chemical formula ZnFeS and an ore of zinc. Ionic Compounds Naming and Formula Writing. 1 NaF is sodium fluoride 2 K2CO 3 is potassium carbonate 3 MgCl 2 is magnesium chloride 4 BeOH 2 is beryllium hydroxide 5 SrS is strontium sulfide 6 Cu 2S is copper I sulfide 7 ZnI 2 is zinc iodide 8 Ca 3PO 42 is calcium phosphate 9 NH 4I is ammonium iodide 10 MnNO 33 is manganese III nitrate.
Ionic Compounds Naming and Formula Writing. Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. When the iron content is high sphalerite is an opaque black variety called marmatite.
Here we report an indium sulfide catalyst that is stabilized by adding zinc in the structure and shows dramatically improved stability. Zinc sulfide or zinc sulphide is an inorganic compound with the chemical formula of ZnS. It is found in air soil and water and is present in all foodsPure zinc is a bluish-white shiny metal.
Zinc and copper sulfide surfaces are formed in adhesive interlayer during rubber vulcanization. Solubility Product Constants near 25 C. Copy this to my account.
The names are found by finding the intersection between the cations and anions. KCIO potassium hypochlorite 48. Complete these in lab and on your own time for practice.
Compound Name Type of Compound. Harrys also offers a regular Anti-Dandruff formula with 1 percent zinc pyrithione Not only does this sulfate-free shampoo relieve itching and flaking it only costs 7 for a sizable 14. Compound Interest Formula Online.
In some respects zinc is chemically similar to magnesium. Al2S3 aluminum sulfide 54. Zinc is one of the most common elements in the earths crust.
ZnCH3COO2 zinc acetate 58. Sodium thiosulfate ____Na2S2O3_____ 3. PbO2 lead IV oxide 56.
The chemical symbol for Zinc is Zn. ZnBr2 zinc bromide 45. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
Centroid Of Trapezoid Formula. Mg3N2 magnesium nitride 49. Fe2CrO43 iron III chromate 46.
Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound. The amount of iron substitution that occurs depends upon iron availability and temperature with higher temperatures favoring higher iron content.
Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box. Sulfur dioxide ____SO2_____ 2. Mean And Standard Deviation Formula.
Formula to name problems. Zinc sulfate is the inorganic compound with the formula ZnSO4 and historically known as white vitriol. Ionic Compound Formula K sp.
Calculate the empirical formula of copper sulfide. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3.
1 separation of an immiscible sulfide melt during the early stages of crystallization of basic magmas. Sulfide mineral deposits originate in two principal processes both of which have reducing conditions. German geologist Ernst Friedrich Glocker discovered sphalerite in 1847 naming it based on the Greek word sphaleros meaning deceiving due to the difficulty of identifying the mineral.
This is the main form of zinc found in nature where it mainly occurs as the mineral sphalerite. A zinc and copper alloy is used to make pennies in the United States. K2SO4 potassium sulfate 59.
E-mail to a friend. Selenium sulfide shampoos are associated with a higher incidence of adverse effects than ketoconazole with the most common reactions being. Empirical Formula Hill Notation.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. In its dense synthetic form zinc sulfide can be transparent and it is. Perimeter Of Rectangle Formula.
It is a zinc sulfide containing variable amounts of iron that substitutes for zinc in the mineral lattice. The following data were obtained. SnSO42 tin IV sulfate 55.
Chemical Product and Company Identification Product Name. Potassium nitrate sulfur and carbon Gunpowder Oxygen and water Sea foam.
Answered A Piece Of Magnesium Metal Is Dropped Bartleby
Phosphorus is used in photosynthesis and overall.

Magnesium & zinc nitrate. Magnesium a mineral that supports immune heart. Zinc oxide is an inorganic compound with the formula Zn OZnO is a white powder that is insoluble in water. The soil does not need to.
Therefore none of zinc copper or lead will displace magnesium ions from solution so that there will be no reaction. 02ppm DTPA extractable Cu Designed and Developed by National Informatics Centre Information and data in this application is managed by State Agricultural Departments and Department of Agriculture and Farmers Welfare STCR. Cium Ca magnesium Mg boron B chlorine Cl copper Cu iron Fe manganese Mn molybdenum Mo nickel Ni and zinc Zn.
These 17 essential elements also called nutrients are often split into three groups fig. The negative ion anion is written second in the name. Nitrogen and sulfur are essential to the supply of amino acids and proteins.
Potassium nitrate may increase the hyperkalemic activities of Magnesium sulfate. It is used as an additive in numerous materials and products including cosmetics food supplements rubbers plastics ceramics glass cement lubricants paints ointments adhesives sealants pigments foods batteries ferrites fire retardants and first-aid tapes. 06 ppm DTPA extractable Zn Boron.
Polyatomic ions are ions which consist of more than one atom. The authors noted that the ability of beetroot juice to lower blood pressure depends on the nitrate. The essential nutrients used include calcium nitrate potassium sulphate potassium nitrate mono potassium phosphate and magnesium sulphate.
Isnt it interesting to consider how science is all around us. Zinc Acetate is made from zinc nitrate and acetic anhydride. Hydrogen forms water by combining with the oxygen.
Magnesium MSDS Section 1. Zn 3 PO 4 2. Zinc copper and lead do not react with magnesium nitrate because in the activity sequence magnesium is higher because it is more reactive than other metals.
This form may aid in reducing the duration of the common cold JRSM Open. TBHQ Tertiary Butyl Hydro Quinone. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atoms.
The best way to learn new. Zinc which promotes wound. Magnesium is the only metallic component of chlorophyll.
20ppm DTPA extractable Mn Copper. This is our newest publication and has been created to support the school technician profession in Scotland. 05 ppm hot water soluble boron Iron.
Mangafodipir may decrease the excretion rate of Potassium nitrate which could result in a higher serum level. Potassium nitrate enriched with micronutrients. Magnesium ribbons turnings or sticks Chemical Name.
Zinc acetate is another chemically-altered form of zinc and considered to be more absorbable than gluconate. Potassium nitrate enriched with Zinc. Magnesium trisilicate may decrease the excretion rate of Potassium nitrate which could result in a higher serum level.
Magnesium is a chemical element with the symbol Mg and atomic number 12. Zinc picolinate and zinc gluconate are forms of dietary supplements that we use to prevent zinc deficiency. ZINC ZIRCONIUM finer than 100 mesh.
SLM4408 SLM2263 SLM3637 CAS. Its in the food we eat and the air we breathe. The atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit.
Potassium nitrate enriched with Magnesium. CHARCOAL AND LAMPBLACK any mesh size The following ignition material is restricted to 25 ft per. Potassium Nitrate enriched with Boron.
DC Directly Compressible Calcium Carbonate. Zinc aluminum magnesium and copper Zamak Advertisement Science Is All Around Us. Finally there are seven elements that plants need in tiny amounts.
TITANIUM ALLOYS all are coarser than 100 mesh MAGNESIUM AND MAGNESIUM ALLOY S 10 to 100 mesh with or without 50 Mg chips ALUMINUM POWDERS 100 mesh or coarser. 45ppm DTPA extractable Fe Manganese. 2X DMSO 999 Pharma Grade No Odor - Dimethyl.
Adding Epsom salts diluted to 85 oz. Amyl Nitrate Strange Connection Dirty Bastard Remix by Adriano. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website.
Magnesium deficiency can be misidentified as zinc or chlorine deficiency a virus or as natural aging so notice the details on your plants and cross-examine it with the symptoms on this list. And the more we experiment with it the more we uncover various truths about it. Without it plants cant process sunlight.
To learn more about chemical formula nomenclature of organic compounds and other topics related to chemical formula in chemistry download BYJUS The Learning App. Potassium nitrate enriched with Sulfate. All group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.
It is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column group 2 or alkaline earth metals of the periodic table. Healthy soil that is high in organic matter usually contains adequate amounts of each of these micronutrients. Lead Lux Magnesium Manganese Molybdenum Nickel Nitrate Nitrite Nitrogen ORP Ozone pH Phosphate Phosphorus Potassium Refractometry Relative Humidity Resistivity Salinity Silica Silver Sodium salt Sulphate Sulphide Sulphite Sulphur Dioxide Tartaric Acid Temperature Titration Turbidity Zinc.
You can use this chart to predict whether or not an atom can bond with another atomThe charge on an atom is related to its valence electrons or oxidation stateAn atom of an element is most stable when its outer electron shell is completely filled or half-filled. The positive ion cation is written first in the name. The first group is the three macronutrients that plants can obtain from water air or both carbon C hydrogen H and oxygen O.
Sulfur is a component of many proteins. 30 Count Pack of 1 48 out of 5 stars 876. 98 073Count Get it as soon as Thu Dec 2.
Benefits The high levels of nutrients in turnip greens can enhance health and. FREE Shipping on orders over 25 shipped by Amazon. Probiotic Vitamin C Vitamin D and Zinc Elderberry Immune Support for Kids Mixed Berry Chewables 30 Count.
Turnip greens also contain more than 250 mg of nitrate levels for every 100 grams of leaf which is a very high level. Jicama is packed with nutrients and may provide various health benefits including improved digestion weight loss and a reduced risk of disease. Test your Knowledge on Chemical formula.
Each element involved in these nutrients provides a different benefit. 2017 as well as offer relief for Wilson. AMMONIUM NITRATE POTASSIUM CHLORATE POTASSIUM NITRATE.
Of water or crushed dolomitic limestone to the soil can help address magnesium deficiencies. Put your understanding of this concept. This is a chart of the most common charges for atoms of the chemical elements.
It is used as a catalyst in. 13 They exhibit a slow setting time 6 shrinkage on setting solubility 158 235 and they can stain tooth structure.

The Biological Inorganic Chemistry Of Zinc Ions Sciencedirect
Noncombustible but accelerates the burning of combustible materials.
Zinc nitrate solubility. Toxic oxides of nitrogen are produced in fires involving this material. Compare the rate of reaction between zinc and sulfuric acid with copper as a catalyst in this simple class practical. Even the nitrate products are oxidizing agents.
Prolonged exposure to fire or heat may result in an explosion. 1 Nickel IIIchloride potassium phosphate -- Molecular equation. The reaction between metallic zinc and hydrogen chloride gas yields the anhydrous form of zinc chloride.
Catalysts for the thermal decomposition of potassium chlorate. Method II Ion. The mean plasma zinc area under the curve for zinc.
It is soluble in both water and alcohol. K sp M n m A m- n. Zinc nitrate is an inorganic chemical compound with the formula ZnNO 3 2.
Zinc zn2 lead ii pb2 aluminum al3 fluoride f- soluble slightly soluble soluble soluble insoluble insoluble slightly soluble slightly soluble slightly soluble soluble soluble soluble insoluble slightly soluble chloride cl- soluble soluble soluble soluble soluble soluble soluble soluble soluble soluble insoluble soluble insoluble soluble bromide br- soluble soluble soluble. However the microemulsion was prepared from ZnNO 3 2 cyclohexane acrylonitrile-butadiene-styrene copolymer ABS butanol and hydrogen peroxide H 2 O 2. Whether or not such a reaction occurs can be determined by using the solubility rules.
All processes were carried out in a reactor. An early zinc. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website.
Zinc hydroxide Zn 2 is an inorganic chemical compoundIt also occurs naturally as 3 rare minerals. Magnesium nitrate is a crystalline source with high water solubility for use consistent with nitrates and lower acidic pH. Like the hydroxides of other metals such as lead aluminium beryllium tin and chromium Zinc hydroxide and Zinc oxide is amphotericThus it will dissolve readily in a dilute solution of a strong acid such as HCl and.
If large quantities are involved in a fire or the combustible material is finely divided an explosion may result. It is important to note that the zinc-chlorine bond in ZnCl 2 possesses some covalent characteristics which accounts for its low melting point and its solubility in ethereal solvents. Metallic zinc is an ideal anode material for aqueous batteries but suffers from irreversibility issues.
Beginning with Volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of solubilities of gases liquids and solids in binary. Method I Thorium Nitrate Colorimetric Method. State the method you would choose to prepare the following salts.
After fertilization crops take up a relatively small part of added nitrogen compounds namely 25-30. In association with Nuffield Foundation. The most widely applied nitrogen fertilizers are probably NaNO 3 sodium nitrate and NH 4 NO 3 ammonium nitrate.
Wülfingite orthorhombic ashoverite and sweetite both tetragonal. For ionic compounds with limited solubility in water an equilibrium constant K sp can be defined from the ion concentration in water from the equation. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution. Cd 3 AsO 4 2. 75 320 An advantage to this sealer group is antimicrobial activity.
A solidsolid reaction between lead nitrate and potassium iodide. A sodium carbonate b calcium chloride c barium sulphate d leadII nitrate e leadII chloride. The residue ends up in groundwater and surface water through soils because nitrates are water soluble.
Under either high pH 5 or low pH 3 intragastric pH conditions. Where M m A n is the slightly soluble substance and M n and A m-are the ions produced in solution by dissosiation of M m A n. Melting point - the temperature at which a solid turns into a liquid.
Zinc oxideeugenol sealers will absorb if extruded into the periradicular tissues. Organic fertilizers mainly contain nitrogen as proteins urea or. This is our newest publication and has been created to support the school technician profession in Scotland.
The solubility of zinc salts is affected by gastric pH. Nitrate compounds are usually soluble in water. Back to top Citing this page.
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. In the mid-1970s a group of chemists and chemical engineers came together within IUPAC to exhaustively compile and critically evaluate reports of experimental measurements of solubility primary chemical literature. The nitrate compounds can form a flammable mixture when combined with hydrocarbons.
The chemical equation for. An aqueous electrolyte based on Zn and lithium salts using either LiMn2O4 or O2 cathodes now. Healthy subjects were given a single oral dose of 50 mg elemental zinc as the acetate.
Zinc salts are not equal in solubility which is important in zinc absorption. Organic Ions and Compounds. List of 131 compounds in the Solution Calculator database for which the density function is defined are for the Perrys Chemical Engineers Handbook.
Try this demonstration to investigate the effectiveness of. Zinc oxideeugenol sealers have a history of successful use over an extended period of time. Use solubility rulesactivity tables and tables for strong bases and acids to write the equations.
Boiling point - the temperature at which a liquid turns into a gas. The emulsion consisted of ZnNO 3 2 and an appropriate surfactant cationic anionic or non-ionic. Zinc nitrate is a colorless crystalline solid.
Includes kit list and safety instructions. This white crystalline salt is highly deliquescent and is typically encountered as a hexahydrate ZnNO 3 2 6H 2 O. In all of these methods the starting solution used was zinc nitrate ZnNO 3 2.
One way of controlling cadmium in effluent streams is to add sodium hydroxide which precipitates insoluble. A potassium nitrate b zinc nitrate c magnesium sulphate d copperII carbonate. Ksp solubility product constants of many popular salts at SolubilityOFthings.
Cadmium is a highly toxic environmental pollutant that enters wastewaters associated with zinc smelting Cd and Zn commonly occur together in ZnS ores and in some electroplating processes. Perry Perrys Chemical Engineers Handbook McGraw-Hill 2 99-118 2008. M m A n s mM n aq nA m-aq.
State whether the following salts are soluble or insoluble. The table below gives calculated values of K.
And the more we experiment with it the more we uncover various truths about it. Water has a high specific heat.
The color change in phenolphthalein is a result of ionization and this alters the shape of the phenolphthalein molecules.

Zinc nitrate physical properties. 2017 as well as offer relief for Wilsons disease a genetic disorder whereby the body stores toxic levels of copper. Nitrogen is an essential element required for successful plant growth. Other applications are in dentistry and in high-capacity zinc long-life batteries.
The rosin increases fracture resistance and the zinc acetate is effective in accelerating the reaction rate. The liquid is a preparation of eugenol which reacts with the powder to form an amorphous chelate of zinc eugenolate. He then made a solution of didymium nitrate and added ammonium hydroxide.
Toxic oxides of nitrogen are produced in fires involving this material. It is also soluble in acetone ethanol and glycerol. 212 contains zinc oxide rosin and zinc acetate in the powder.
The four polymorphs of ZnCl 2 feature a tetrahedral coordinate geometry between the Zn 2 ions and the Cl Molten zinc chloride is. Magnesium nitrate MgNO32 - Magnesium nitrate is the chemical name of MgNO32. Zinc nitrate is a colorless crystalline solid.
If large quantities are involved in a fire or the combustible material is finely divided an explosion may result. Visit BYJUS to understand the properties structure and uses of Magnesium nitrate. In materials science zinc oxide is classified as a semiconductor in group II-VI whose covalence is on the boundary between ionic and covalent.
Its in the food we eat and the air we breathe. By the way if its not actually the holidays and I havent updated this recently sorry about that. Zinc acetate is another chemically-altered form of zinc and considered to be more absorbable than gluconate.
Silvers catalytic properties make it ideal for use as a catalyst in oxidation reactions. The synthesis is typically carried out at temperatures of about 90 C in an equimolar aqueous solution of zinc nitrate and hexamine the latter providing the basic environment. Silver levels in soil are not usually high except in mineral-rich areas when they can sometimes be as much as 44 ppm.
Although inorganic nitrogen compounds ie NH 4 NO 2 and NO 3 account for less than 5 of the total nitrogen in soil they are the main form of the element absorbed by most plantsInorganic and organic fertilizers are applied to maintain the nutritional condition of different cropping systems. That is both solute and solvent can be recovered in chemically unchanged forms using appropriate separation methods. This form may aid in reducing the duration of the common cold JRSM Open.
This classification relates to the dependency of the properties upon the size or extent of the system or object in question. Specific heat is the amount of energy required to change the temperature of a substance. In alkaline it turns pink.
Physical properties of materials and systems are often described as intensive and extensive properties. Plants can absorb silver and. The longer involves a recap of the properties of metals.
Zinc Acetate is made from zinc nitrate and acetic anhydride. Zinc chloride is solid at room temperature and has a white crystalline appearance. He concentrated his attention on the first precipitate and measured its spectrum which revealed it to be a new element samarium.
Zinc acetate is the best. The toxicity of silver was significantly less in the zinc-pretreated cells. Zinc is a bluish-white lustrous diamagnetic metal though.
Samarium itself was eventually to yield other rare-earths. Potassium nitrate sulfur and carbon Gunpowder Oxygen and water Sea foam. Certain additives such as polyethylene glycol or polyethylenimine can improve the aspect ratio of the ZnO nanowires.
Isnt it interesting to consider how science is all around us. Black powder is. Water has several other unique physical properties.
I promise that I wont take your. Im glad you found your way here for chemistry help because youre in the right spot to be enlightened. For example solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate.
Gadolinium in 1886 and europium in 1901. Here are the rules for using this free website. Zinc aluminum magnesium and copper Zamak Advertisement Science Is All Around Us.
To be suitable for use water must be free from all impurities that are offensive to the sense of sight taste or smell and one very important physical characteristic that should be encountered when discussing water quality is turbidity Davis and Cornwell 2012. Its a blend of potassium nitrate saltpeter charcoal and sulfur in a 751510 ratio. A zincblende unit cell.
The nitrate ZnNO 3 2 chlorate ZnClO 3 2 sulfate ZnSO 4 phosphate Zn 3 PO 4 2 molybdate ZnMoO 4 cyanide ZnCN 2 arsenite ZnAsO 2 2 arsenate ZnAsO 4 2 8H 2 O and the chromate ZnCrO 4 one of the few colored zinc compounds are a few examples of other common inorganic compounds of zinc. Your Post-apocalypse Chemistry Connection. As a result pure.
An intensive property is a bulk property meaning that it is a physical property of a system that does. Prolonged exposure to fire or heat may result in an explosion. In acidic solutions it is colorless.
It is used as a catalyst in. The phenolphthalein indicator allows chemists to visually identify whether a substance is an acid or a base. Physical properties of water are related to the appearance of water namely the color temperature turbidity taste and odor.
Noncombustible but accelerates the burning of combustible materials. The formation of a solution from a solute and a solvent is a physical process not a chemical one. Physical properties are used to observe and describe matter.
Because water has a high specific heat it can absorb large amounts of heat energy before it begins to get hot. He observed that the precipitate which formed came down in two stages. Zinc oxide with its unique physical and chemical properties such as high chemical stability high electrochemical coupling coefficient broad range of radiation absorption and high photostability is a multifunctional material 12.
The zinc oxideeugenol cements are used to provide a sedative. Doping of the. The solubility of this compound in water corresponds to 432g100g.
Water in a pure state has a neutral pH. Following pretreatment with zinc chloride hepatocytes were treated with 20 or 40 uM silver nitrate for 24 hr and cytotoxicity was then assessed by enzyme leakage and loss of intracellular potassium. Zinc oxideeugenol cement Fig.
The best way to learn new. Physical Science Text. One of the key ingredients for firecrackers ground fireworks and aerial fireworks ones which explode in the sky is black powder invented by the Chinese about 1000 years ago.
Silver in the environment. Physical Properties of Zinc chloride.
It is a conjugate acid of a nitrate. Animal Pak Vitamin Pack Supplement Zinc Vitamin C B D Amino Acids and More Sports Nutrition Supplement Convenient All-in-One Packs 44 Packs 2698 061Count In Stock.
What It The Chemical Equation For The Reaction Between Zinc And Nitric Acid Quora
23 offers from 1399.
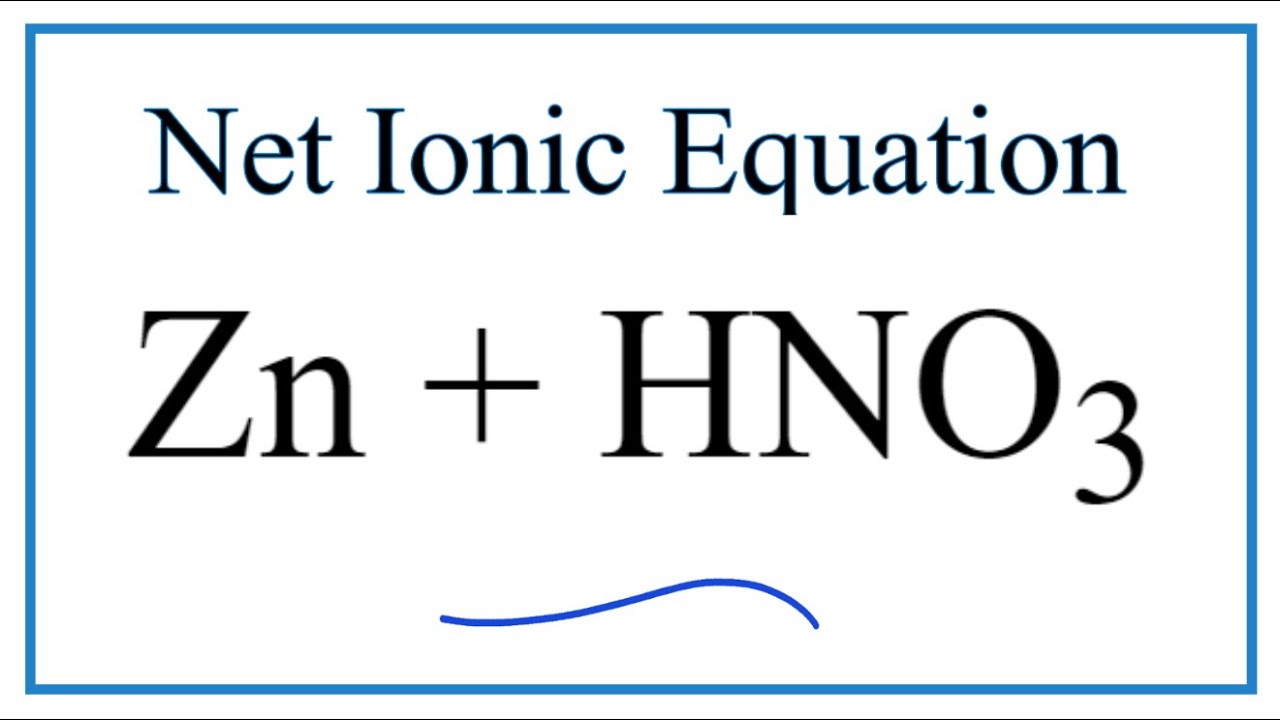
Zinc and nitric acid. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. Extremely stable for storage at room temperature. Hydrochloric acid nitric acid sulphuric acid hydrobromic acid hydroiodic acid perchloric acid and chloric acid are the solid acids.
Walnuts an excellent source of omega-3 fatty acids are known to boost dopamine and arginine levels in the brain which increases the production of nitric oxide. The level at which zinc. This is our newest publication and has been created to support the school technician profession in Scotland.
Symptoms of short-term nitrogen dioxide exposure include nausea dyspnea and headache. Nitric oxide is the essential chemical compound for erections. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes.
The Association for Academic Surgery is widely recognized as an inclusive surgical organization. NO is a gaseous signaling molecule. Nitric acid is reduced in to nitric oxide NO.
The solution can be reduced using zinc and an acid - either hydrochloric acid or sulphuric acid usually using moderately concentrated acid. Aluminum calcium lanthanum nickel strontium tungsten antimony chromium lithium potassium tellurium vanad ium arsenic cobalt magnesium. 7300 Issue 3 EVALUATION.
The exact vanadium ion present in the solution is very complicated and varies with the pH of the solution. Nitric acid 0-30 S S I Nitric acid 30-50 S O Inks S S Nitric acid 70 S O Iodine crystals O O Nitric acid 95-98 U U Isobutyl alcohol S S Nitrobenzene 100 U U Isopropyl alcohol S S Nitroglycerine O U Isopropyl ether O U O K Octane S S Kerosene O O Oleum concentrated U U Olive oil S S L Orange juice S S Lactic acid 10 S S Oxalic acid dilute S S Lactic acid 90 S S Oxalic acid. As seen in the acid deposition section nitric oxide can transform into nitrogen dioxide this can happen with the hydroperoxy radical HO 2 or diatomic oxygen O 2.
Much more sensitive than EtBr SYBR Safe and others. The pure compound is colorless but older samples tend to acquire a yellow cast due to decomposition into oxides of nitrogen and water. Non-mutagenic for safer handling and easy disposal.
Nitric acid is a nitrogen oxoacid of formula HNO3 in which the nitrogen atom is bonded to a hydroxy group and by equivalent bonds to the remaining two oxygen atoms. Zinc is an essential micronutrient for humans and is extensively involved in protein lipid nucleic acid metabolism and gene transcription1 Its role within the human body is extensive in reproductive function immune function wound repair and at the microcellular level on macrophage neutrophil natural killer cell and complement activity234 Despite being one of the most. Simple precast or post-electrophoresis.
To learn more about hydrochloric acid and its reaction with sodium thiosulfate download BYJUS The Learning App. Zinc sulfide and nitric acid reaction. Puritans Pride L-arginine 1000 Mg Capsules 100.
Equivalent Weight of Oxalic Acid Calculation The molar mass of hydrated oxalic acid is 126 grams per mole. GelGreen is a highly sensitive non-toxic green fluorescent nucleic acid dye designed for staining DNA in agarose gels. 6 offers from 2119.
15 August 1990 Issue 3. 43 out of 5 stars 35012 1 Best Seller in L-Arginine Nutritional Supplements. About the Societies.
Beets rarely rank high on anyones list of most-loved vegetables. Oxalic acid is produced by the action of either hydrate of potash or of nitric acid upon most organic compounds of natural occurrence. It is also called diprotic acid.
15 March 2003 OSHA. Sulfide ion is oxidized to sulfate ion. 45 out of 5 stars 3472.
NitricPerchloric Acid Ashing MW. Havasu NutritionL-Arginine Endurance and Circulation Booster with Nitric Oxide 60 Caps. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses.
But heres a reason to give these ruby-red roots another try. Although since 1983 pennies are actually made of zinc surrounded by a paper-thin copper foil to give them the traditional appearance of pennies Copper is oxidized by concentrated nitric acid HNO 3 to. Beets contain naturally high levels of nitrates which your digestive system converts into nitric oxide.
Interestingly rats pre-treated for 3 d with a high dose of zinc 30 mgkg before colitis was induced via intrarectal DNBS dinitrobenzene sulfonic acid showed significantly fewer TJs that were leaky to lanthanum and gained more weight than controls but a lower dose of zinc did not show this effect despite still being 10 times the daily requirement for humans. Now Foods Supplements L-Arginine 500 mg Nitric Oxide Precursor Amino Acid 250 Count 00176 Ounce. Most commercially available nitric acid has a concentration of 68 in water.
It dilates the blood vessels allowing blood to travel freely. The ion is usually. Copper Nitric Acid.
The reaction is done under acidic conditions when the main ion present is VO 2 - called the dioxovanadiumV ion. Long-term effects could include impaired immune and respiratory function. Reaction with barium chloride yields a white precipitate that is insoluble in hydrochloric acid and in nitric acid.
It has a role as a protic solvent and a reagent. When the solution contains more than 86 HNO 3 it. Copper is a reddish-brown metal widely used in plumbing and electrical wiring.
Since the chemical formula of this compound can be written as COOH-COOH it can be understood that oxalic acid is a. So the white precipitate ZnS is disappeared when reaction is completed because ZnSO 4 is soluble in water. The impetus of the membership remains research-based academic surgery and to promote the shared vision of research and academic pursuits through the exchange of ideas between senior surgical residents junior faculty and established academic surgical professors.
Nitric acid magnesium oxide magnesium nitrate water Acids and metal carbonates When acids react with carbonates such as calcium carbonate found in chalk limestone and marble a salt. Take up a quiz on Hydrochloric acid. Beet juice may help lower blood pressure according to a study in the February 2015 Hypertension.
Reaction with lead acetate yields a white precipitate that is soluble in ammonium acetate sulfate test. Nitric acid H NO 3 also known as aqua fortis Latin for strong water and spirit of niter is a highly corrosive mineral acid. Put your understanding of.
The mixture containing one volume of concentrated nitric acid and three volumes of concentrated hydrochloric acid is known as aqua regia. It is perhaps most familiar to people in the United States in the form of the penny.