General preparation of polyoxygen difluorides. O element oxygen-based compounds Atomic number.
Corrosive to skin and eyes.

Oxygen difluoride formula. When the compound contains oxygen and a halogen the name of the halogen is the first word in the name. Calculate empirical formula from data giving composition by mass or percentage by mass. Following are several physical.
1 for the 021-371 μm wavelength range. Write the formula for each compound. It is a member of the chalcogen group in the periodic table a highly reactive nonmetal and an oxidizing agent that readily forms oxides with most elements as well as with other compounds.
Physical Properties of Noble Gases. Formula of Ionic Compound s 2. For example we have already seen CH 4 the molecular formula for.
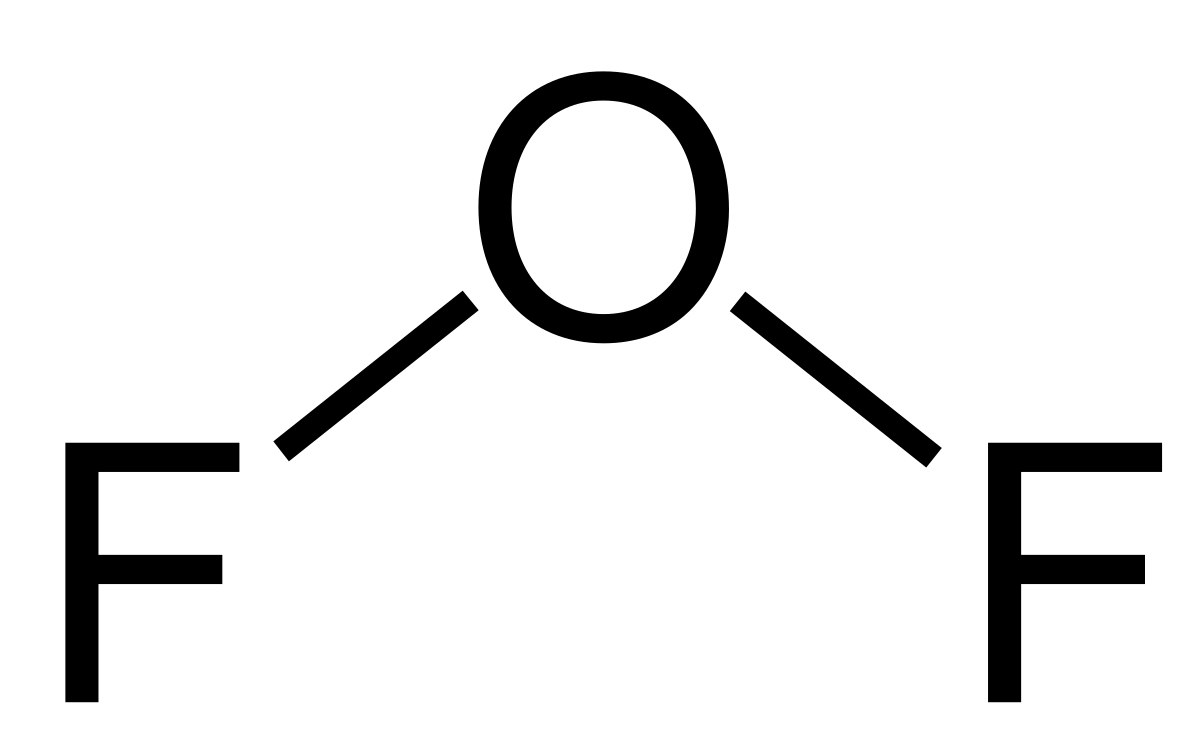
Empirical and molecular formula. El difluoruro de oxígeno es un compuesto químico con la fórmula OF 2Según la teoría TREPEV su estructura es similar a la del agua sin embargo tiene propiedades muy diferentes a estaAl tener flúor y oxígeno los elementos más electronegativos que existen este compuesto es un fuerte oxidante. If both elements are in the same group the element with the higher period number is written first in the name.
Hydrogens oxidation number is 1 excluding when it is bonded to metals containing two elements. Nitrogen Monoxide Nitrogen Trifluoride. It was obtained by the electrolysis of molten potassium fluoride and hydrofluoric acid containing.
It is considered as a powerful fluorinating agent. Prolonged exposure of the containers to high heat may result in their violent rupturing and rocketing. Then the other nonmetal symbols are listed.
Chemists usually list this central atom first in the chemical formula. In H 2 O for example there is a bonding pair of electrons between oxygen and each hydrogen. 검색 000000 Products Filtering Condition.
Shipped as a liquefied gas under its own vapor pressure. PF5 has the same number of fluorine atoms as 250 g of oxygen difluoride OF2. For example CaH 2 its oxidation number equals to 1.
The following diagrams represent the reaction of A2 shaded spheres with B2 unshaded spheres. A common preparative method involves fluorination of sodium hydroxide. Boiling Point BP Oxygen changes its state from liquid to gas at -18296C -297328F or 9019K Oxygen is a colorless odorless and tasteless gas.
Typically a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table except that hydrogen is almost never written first H 2 O is the prominent exception. IronII fluoride or ferrous fluoride In Fe2O3 there are 3 O 2-ions per formula unit total charge. Oxygen difluoride appears as a colorless poisonous gas with a strong peculiar odor.
Write the formula for each compound. It has been reported to be produced from atomic fluorine and dioxygen. Students should be able to.
What volume of a 0540 M NaOH solution contains 115 g of NaOH. F 2 SO 2 or SO 2 F 2 or F 2 O 2 S. Oxygen Difluoride P2O3 Diphosphorus Trioxide P2O5 Phosphorus Pentoxide P4O10 Phophorus Pentoxide P4S3 Phosphorus Sesquisulfide PbC2H3O22 LeadII Acetate PbC2H3O24 LeadIV Acetate PbNO32 LeadII Nitrate PbCl2 LeadII Chloride PbCl4 LeadIV Chloride PbCrO4 LeadII Chromate PbI2 LeadII Iodide PbO LeadII Oxide PbO2 LeadIV Oxide PBr3 Phosphorus Tribromide PbS.
50 mgm³ quartz tripoli. Oxygen difluoride was first reported in 1929. However it has very different properties being a strong oxidizer.
Mixtures of hydrogen carbon monoxide or methane and oxygen difluoride are exploded when a spark is discharged Mellor 2 Supp. What is the likely formula for the binary compound formed from the elements represented by letters A and D on the periodic table above. Shapes of simple molecules and ions.
In formula F211 Ci denotes the cross-section of O 3 at wavelength interval i Qi denotes quantum yield of O 1 D from O 3 at wavelength interval i Fi denotes the actinic flux of wavelength interval i and n denotes the total number of wavelength intervals relevant to the reaction. As predicted by VSEPR theory the molecule adopts a bent molecular geometry similar to that of water. Ca 25 mgm³ cristobalite tridymite.
Sulfuryl fluoride appears as a colorless odorless gas. Oxygen is the chemical element with the symbol O and atomic number 8. Numerical subscripts are used if there is more than one of a particular atom.
Principle for a formula unit. Ammonia Exposure Routes inhalation skin andor eye contact Symptoms Cough dyspnea breathing difficulty wheezing. 15999 Ionization energy eV.
It has low vapor pressure and is sensitive to moisture. Xenon Difluoride XeF 2 is a dense white crystalline solid having a linear geometry. Name of Ionic Compound.
2 Xenon Fluorides. Write the formula for each compound. If any electrons are left over place them on the central atom.
Capitalization and subscripts are graded. Decreased pulmonary function progressive. An explosion occurred upon heating 1-pentol and 1-pentol under hydrogen pressure.
Can explode on contact with waterDecomposes to toxic gaseous fluorine if heated to high temperature. Oxygen difluoride OF 2. It appears that this acetylenic compound under certain conditions suddenly breaks down to form elemental carbon hydrogen and carbon monoxide with the release of.
These electrons will usually be lone pairs. 15999 Ionization energy eV. SeF4 OF2 N2O PCl3.
Oxygen is bonded to fluorine- Example dioxygen difluoride where the oxygen atom is allocated an oxidation number of 1. In FeF2 there are 2 F-ions per formula unit net charge of -2 so the charge on the one Fe must be 2 the ion is Fe2 Name. While cross-sections and quantum yields may be determined via laboratory experiments actinic fluxes.
Sellmeier formula is reported in Ref. Boiling Point BP Oxygen changes its state from liquid to gas at -18296C -297328F or 9019K Oxygen is a colorless odorless and tasteless gas. 2 verifies the validity of the formula up to 67 μm.
O element oxygen-based compounds Atomic number. Fluoroperoxyl is a molecule such as O-O-F whose chemical formula is O 2 F and is stable only at low temperature. Reaction equation F 2O 2 by volume.
Xenon can bring up to its highest oxidation state of 8 only by the one element that is known as oxygen. Fluorine chlorine trifluoride manganese trioxide oxygen difluoride hydrogen peroxide etc. Sulfuric acid 98072 g.
Molecular formula is the actual number of atoms of each element in a compound. Beginning with the terminal atoms add enough electrons to each atom to give each atom an octet two for hydrogen. Data Expressions for n CSV - comma separated TXT - tab separated Full database record Optical transmission calculator.
At standard temperature and pressure two. Very toxic by. Fluorine and other halogens have an oxidation number 1 when they appear as halide ions in their compounds.
Highly toxic by inhalation. After hydrogen and helium oxygen is the third-most abundant element in the universe by mass. Simple Binary Ionic Compounds Please complete the following table.
Oxygen difluoride is a chemical compound with the formula OF 2. The second element in the name is named as if it were an anion ie by adding the suffix -ide to the root of the element name eg fluorine F.