As in aqueous solution protons H are preferentially reduced before Al 3 ions leading to hydrogen evolution the reduction of Al 3 requires to perform the electrolysis in a molten aluminium salt in the absence of water. The mass of the anhydrous CaSO4 salt is 2304 g.
This is the salt that is used in food.

Is caso4 an anhydrous salt. It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster blackboardsidewalk chalk and drywallA massive fine-grained white or lightly tinted variety of gypsum called alabaster has been used for sculpture by many cultures including. Sodium carbonate is a salt formed from a strong base and we know that bases turns litmus red to blue. Therefore all precautions shuld be made to minimize errors in the analysis.
The anhydrous compound is a crystalline solid with a melting point of 307. For strongly electropositive metals molten electrolyte is subjected to. Given that the molar mass of the anhydrous.
The anhydrous salt decomposed into elements. Calculate the percent by mass of water in the hydrated calcium sulfate. Is cacl2 soluble in water email protected.
Acetone can be shaken with Drierite 25gL for several. Sodium carbonate and hydrochloric acid reaction type. A Calcium sulfate CaSO4 CAS Reg.
2009 版 材料名称 分子式 折射率 Acanthite Silver Sulphide Ag2S 硫化银 22 Acetal 乙缩醛 148 Acetone 丙酮 136 Adipic Acid CH2CH2COOH2 脂肪酸 1433 Agate SiO2 玛瑙 1544 - 1553 Albite Na2OAI2O36SiO2 钠长石 1529 Albite Feldspar NaAlSi3O8 钠长石 1527 - 1. H 2 SO 4. The water of hydration was released as water vapor.
Anhydrous MgSO4 is an inefficient drying agent and CaCl2 forms an addition compound. The number of molecules of water of crystallisation in the salt is 5. OIL RIG for oxidation and reduction in terms of electrons.
C Sulphuric acid. 1434gThe mass of the anhydrous CaSO4 salt is 2. The gravimetric analysis of this experiment is meant to be quantitative.
Academiaedu is a platform for academics to share research papers. Ca H2SO4 arrow CaSO4 H2. It is produced by the action of chlorine on dry slaked anhydrous but actually contain some water lime.
Calcium Chloride - CaCl 2. Drierite anhydrous CaSO4 offers minimum acid and base catalysis for aldol formation and is the recommended drying agent for this solvent Coetzee Siao Inorg Chem 14 2 1987 Riddick Bunger Organic Solvents Wiley-Interscience NY 3rd edn 1970. What is the effect of temperature on solubility of KNO 3 and CaSO 4 in water.
Go Premium and unlock all our brewing tools. In the same way Solubility of calcium sulphate CaSO4 in water decreases with an increase in temperature. Agno3 SilverI Nitrate Nitric Acid SilverI Salt AgNO3 Molar Mass AgNO3 Oxidation Number.
A solution is. Oxidation is loss reduction is gain. Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate with the chemical formula CaSO 4 2H 2 O.
Drierite anhydrous CaSO4 offers minimum acid and base catalysis for aldol formation and is the recommended drying agent for this solvent Coetzee Siao Inorg Chem 14 2 1987 Riddick Bunger Organic Solvents Wiley-Interscience NY 3rd edn 1970. Molar mass of alcl3. We were trying to determine the mass of the hydrate anhydrous salt and water as well as the empirical.
The formula weight of the anhydrous salt is 160. 7778-18-9 or CaSO4-2H2O CAS Reg. Use memory aids such as.
Acetone can be shaken with Drierite 25gL for several. Molecules attached to them. Upon exposure to fairly dry air it effloresces forming powdery anhydrous sodium sulfate.
Heating mgso4 7h2o. Hydrated salt - Anhydrous salt Hydrated Salt 2914 - 2304 6106102914 209 -- 209. If it would be anhydrous the colour will be white because it wont have water molecule.
Calculate the molarity of nitric acid when 304 ml of a nitric acid solution was titrated with 178 ml of 15 molar KOH. Salt of a strong acid and strong base is neutral with a pH value of 7. With an increase in temperature Solubility of potassium nitrate KNO3 in water increases.
Agcl Agcl SilverI Chloride AgCl Molar Mass Bond Polarity AgCl Oxidation Number. For example if you learn that a carbonate reacts with an acid to form a salt carbon dioxide and water you can write the equation for any carbonate reacting with any acid. Some salt is called rock salts bed of rack salt were formed when seas of bygone ages dried up.
Heat 2 NaHCO 3 ¾ Na 2CO 3 H 2O CO 2 Common salt is an important raw material for various Sodium hydrogen Sodium Water. 5 g of a crystalline salt when rendered anhydrous lost 18 g of water. The anhydrous form is prepared by complete dehydration of gypsum below 300 degC in an electric oven.
Glycine NN-12-ethanediylbis N- carboxymethyl- disodium salt is listed as a High Production Volume HPV chemical 65FR81686. Calcium sulfate is a white solid found as two hydrates a hemihydrate known as plaster of Paris and a dehydrate known as gypsum. 10101-41-4 also known as plaster of Paris anhydrite and gypsum occurs naturally and exists as a fine white to slightly yellow-white odorless powder.
Please use this book to increase your knowledge for the laboratory pratictioner. Peter sometimes collects money at zoos in London helping crazy. The hemihydrate is a white solid as shown in the figure below.
Silver Chloride - AgCl. NaCl common salt is formed by a combination of hydrochloride and sodium hydroxide solution. What would be the colour of litmus in a solution of sodium carbonate.
State the common and chemical names. These water molecules D CaOH2 Cl 2 ¾¾ CaOCl 2 H 2O are called water of crystallisation and such salts are Slaked. The crucible and lid are.
Assume that you prepared phenyl magnesium bromide by reacting 21 mL of bromobenzene density 150 g mL with 050 g of magnesium in anhydrous ether. The anhydrous salts absorbed water to form a hydrate. To this you slowly added a solution of 24 g.
E509 Cacl2 Calcium Dichloride Calcium2 Chloride CalciumIi Chloride Calcium Chloride Anhydrous CaCl2 Molar Mass CaCl2 Oxidation Number. Anhydrous MgSO4 is an inefficient drying agent and CaCl2 forms an addition compound. The common salt thus obtained is an important raw material for various materials of daily use such.
Copper sulphate itself will have no colour due to the absence of water molecule.
The chemical formula for table salt is NaCl. What is the chemical formula for salt.

Product Name Penicillin V D5 Potassium Salt Phenyl D5 Pharmaffiliates Penicillin Potassium Salt
The most common salt that is relevant to our daily lives is table salt which is made up from a Na and a Cl- making its chemical formula NaCl.

What is salt chemical formula. Chemical Formula For Sugar 9. You might know this substance as table salt and it is NaCl. Magnesium sulfate is an inorganic salt with the chemical formula MgSO4H2Oxwhere x varies from 0 to 7.
Since both charges are 1 we drop both numbers and we see that the salt is composed of one lithium atom and one bromine atom. The chemical formula of the salt depends on the acid which represents the source of the anion the valence of the anion and action. The chemical formula of Epsom salt is Magnesium sulfate.
What is the chemical formula of hydrated Epsom salt. Sir Humpty Davy was the chemist who separated the salt in sodium and chloride for the first time in 1807. Salt chemical formula.
Chemical Composition Of Chaka Salt Lake Water River And. Anhydrous monohydrated and heptahydrated form and its chemical formula are. Salt chemical formula.
Ssd solution chemical formula. It is often encountered as the heptahydrate sulfate mineral epsomite MgSO47H2O commonly called Epsom salt. Magnesium sulfate also known as Epsom salt is an inorganic salt mainly used in agriculture as fertilizer.
What is the chemical formula for salt. It is also used by the pharmaceutical industry. Heptahydrate sulphate mineral epsomite MgSO47H2O.
The scientific name for salt is sodium chloride. Table salt molecular formula sodium chloride table salt chemical formula structured. Since table salt is an ionic compound the formula implies that numbers of Na ions and Cl-.
What is ssd solution formula. What is the chemical formula of salt. You must login to add an answer.
For non-molecular substances such as table salt we represent the composition with an empirical formula. Sodium chloride is represented by NaCl meaning that sodium and chlorine ratio in sodium chloride is 1 to 1. Magnesium sulfate has three known form.
Magnesium sulfate chemical formula MgO4S and CAS No. FeCl 3 is the named as iron III chloride while AlCl 3 is aluminum chloride although the valence of iron and aluminum is 3 in the two salts because iron has two valencies II III while aluminum has only. If you are talking about common salt then its formula is NaCl.
If you are talking about common salt then its formula is NaCl. Chemical formula SaltWhat is the chemical formula for saltFacebook. Thoughts Flow For You.
MgSO 4 MgSO 4 H 2 O and MgSO 4 7H 2 O and the molar mass are 120361 g mol -1 138361 g mol -1 and. Common table salt is also called sodium chloride and the chemical formula for salt is Na Cl. Its chemical formula is NaCl.
Basically when we listen salt it may be sea salt also which contains many chemicals but the salt which is used in households is sodium chloride and the chemical formula for this salt is NaCl. What is the chemical formula of salt. Salt is an ionic compound made up of sodium and chloride ions.
7487-88-9 is an inorganic salt chemical compound containing magnesium sulfur and oxygen. Salt consists of one atom of sodium combined with one atom of chlorine. Taking the table salt eqNaCl eq as an example the salt molecule is.
Solved Equation Writing Translate Each Of The Follow Che. The chemical formula for salt is NaCl. Why sodium chemical formula is Na.
In order to derive any salt chemical formula the cation and the anion must first be identified. Some examples of other salts include KCl potassium chloride. Sodium Chloride I Ruishun Trade Co Ltd.
How to get the SSD solution chemical formula. Also called sodium chloride natrium chloride or halite table salt is an ionic compound that contains a positively charged ion of sodium and a negatively charged chloride ion connected through an ionic bond. It can have impurities of gypsum CaSO4 and sylvite KCl but it is very rare to find potassium sulfate as a mineral although occasionally polyhalite K2Ca2MgSO442H2O is It is typically formed by the evaporation of salty water such as sea water.
Sodium chloride commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a. Salt is a thing that we sprinkle on our food give it a taste. What is salt chemical formula.
The chemical Mohrs salt formula of the double salt is FeSO 4NH 4 2 SO 46H 2 O Ferrous Ammonium Sulfate Hexa Hydrate or NH 4 2 FeSO 4 26H 2 O Ammonium IronII SulphateIts name derived from the name of German Chemist Karl Friedrich Mohr who proposed various important approaches of titration in the 19th century. Diy Chemical Formula Salt And Pepper Shaker Set Divas. This is the common name for the mineral halite.
It is clear from the table salt chemical formula that it contains two elements ie. Again the subscript 1 is omitted. The formula H2O is also the molecular formula of water.
A lead or lead equivalent apron is a protective garment which is designed to shield the body from harmful radiation usually in the context of medical imaging. The oxidation number of chlorine in.
All involve inconvenient behavioural properties of the electorlytes and cells themselves.
Flourine lead salt. Ll Doors in horizontal exits shall be openable at all time. I just bought a molecular model kit want to play with my stick and balls 25. Lead has the highest intermolecular forces metallic bonds water and alcohol have hydrogen bonding which are the second strongest bonds here.
2903470000 other perhalogenated derivatives only with flourine chlorine. 30 are dissolved in 250 ml of water. We have provided Pollution of Air and Water Class 8 Science MCQs Questions with Answers to help students understand the concept very well.
Both patients and medical personnel utilize lead aprons which are customized for a wide range of usages. If you discharge any of these metals their compounds or aqueous solutions of their compounds into the sewer system make sure you meet these concentrations. For this purpose steps shall not be used.
At least one of the exits shall lead directly to the exterior or street. F- to attain the electron configuration of nearest noble gas Ne and become a negatively charged anion. At last we have rubidium fluoride which is a salt.
2 READ Dr Sebis Eva Salve Tagged Breast cancer Congestive heart failure Iron flourine Lily of the Valley Potassium phosphate Dr. 2903490000 2903490000 other perhalogenated derivatives with other flourine chlorine. Flourine Magnesium Rubidium Bromine.
Male rats were injected close to the prostate with either 50 µg of lead acetate. Cellulose vermiculite and activated carbon help distribute the heat evenly. Baby if you let me pump my H ions into your intermembrane space it would induce a massive.
05 mole of BaCl2 are mixed with 02 mole ofNa 3 P0 4 the maximum number of mole ofBa 3 P0 4 2. Free PDF download of Class 12 Chemistry revision notes short key-notes for Chapter 7 - The p-Block Elements to score high marks in exams prepared by expert Chemistry teachers from latest edition of CBSENCERT books. Other types of hand warmers use lighter fluid a platinum catalyst helps lighter fluid oxidize exothermically charcoal charcoal oxidizes in a special case or electrical units that produce heat by passing an electrical current from.
A hydrogen atom in a. The molarity of solution is. The sum of the oxidation numbers in.
Even water burn in fluorine with a bright. Notably flourine and chlorine Air-metal molten-salt and molten-metal batteries might afford large-scale capabilities though most research seems to have had limited success. The element has been recorded around 50 ppb in city environments.
2562 g of salt was quantitatively converted to free acid by. SPECTRO brings a complete range of X-ray fluorescence spectrometers XRF. Check the below NCERT MCQ Questions for Class 8 Science Chapter 18 Pollution of Air and Water with Answers Pdf free download.
MCQ Questions for Class 8 Science with Answers were prepared based on the latest exam pattern. C Oxidation number of flourine is -1 in all compounds. Filtering and precipitation for some other type of.
The number of bond pair and lone pair of electrons present in the interhalogen compound BrF 3 is a 1 3 b 3 2 C 5 1 d 7 0 Answer. 06 ppb of fluorine is present as organic chloride compounds and salt spray in the atmosphere. FHF has the highest boiling point of HF HCL and HBr.
Fluorine is reactive with other elements which can combine with nearly any element on Earth. Other Halogens have an oxidation number of -1 in binary compounds unless paired with oxygen which makes them 1 4 The sum of oxidation numbers in all neutral compounds is 0. Is nf3 polar.
Mm Ramps with a slope of not more than 1 to 10 may be substituted for the. Rb to attain electron config of nearest noble gas Xe and form a positive ion. Kk Where there is difference in level between connected areas for horizontal exit ramps not more than 1 in 8 slope shall be provided.
Dielectric Constant k is a number relating the ability of a material to carry alternating current to the ability of vacuum to carry alternating current. On molecular weight polarity and hydrogen bonding. A flourine b chlorine c bromine d iodine Answer.
Example-Name-Munish Designation-Chairman Address-Lucknow Period Of Tenures-25012005 To 21062009 Telephone-123456. This allows AEM outputs to be relevant for the large concentration gradients that can develop during XFC where salt concentrations within the cathode regions can at times approach and exceed 3. Explore the XRF analyzer range here.
The mole abbreviated mol is the SI base unit to measure the amount or quantity of substance. Gingers ability to prevent platelet aggregation or clotting may protect the body from atherosclerosis according to the University of Maryland Medical Center 1 2. The molecular mass of an organic acid was determined by the study of its barium salt.
The 13th most abundant element in the Earths crust is Fluoride. Hydrogen bonds are a relative strong intermolecular force of attraction. Chem students do it on the table periodically 26.
High Blood Pressure Detox Dr. B 3 2. This numerical value is known as the Avogadro constant.
What is a Mole. Lead mercury and silver are especially important pollutants. 2903520000 2903520000 other halogenated derivatives of.
Lead 20 mgl Mercury 002 mgl Silver 04 mgl. Br- to attain the same electron configuration of the nearest noble gas Kr and form a negatively. 3 g of a salt m.
The oxidation number of oxygen in F 2 O is a -2 b -1 c 2 d 1 Answer. Confusion between intrinsic and apparent half-life values can lead to misinterpretation of biomonitoring data and can result in exaggerated predictions in subsequent modeling efforts. Its used as a standard measurement for a very large quantity of very small units of matter.
AEM has been designed to remain accurate over salt concentration out to the onset of solid-phase formation which will manifest differently depending on the choice of salt solvents and temperature. You be Flourine and Ill be Francium and maybe later I can give you an electron 24. One mole is equal to 602214076 10 23 elementary entities such as atoms molecules or ions.
This work provides a review of the first-order equations that have been developed to calculate intrinsic and apparent half-life values and the potential bias that can result from confusing these two values. Certain Facts About Fluorine. Salt in the hand warmer catalyzes the reaction so it produces heat more rapidly.
As is the case with many protective garments it is important to remember that a lead apron is only effective when it is worn. Lead acetate was also a promoter of 2-ethylnitrosaminoethanol-induced renal carcinomas in Fischer 344 rats Shirai et al 1984. The list of Dr.
Mg2 to attain electron config of Ar and form a positively charged cation. Lead in the form of lead acetate was found to accelerate the onset and development of all renal lesions in rats exposed to N-4-fluoro-4-biphenylacetamide Tanner and Lipsky 1984. Lead mercury and silver require special precautions for disposal.
If it is insoluble in water take sodium carbonate extract and add a few drops of sodium nitroprusside solution. Sulphide S 2- Take the water extract and add sodium nitroprusside to it.
Lead Sulphide Precipitate 2 Of 3 Stock Image C036 3892 Science Photo Library
Shipped as a liquid confined under its own vapor pressure.
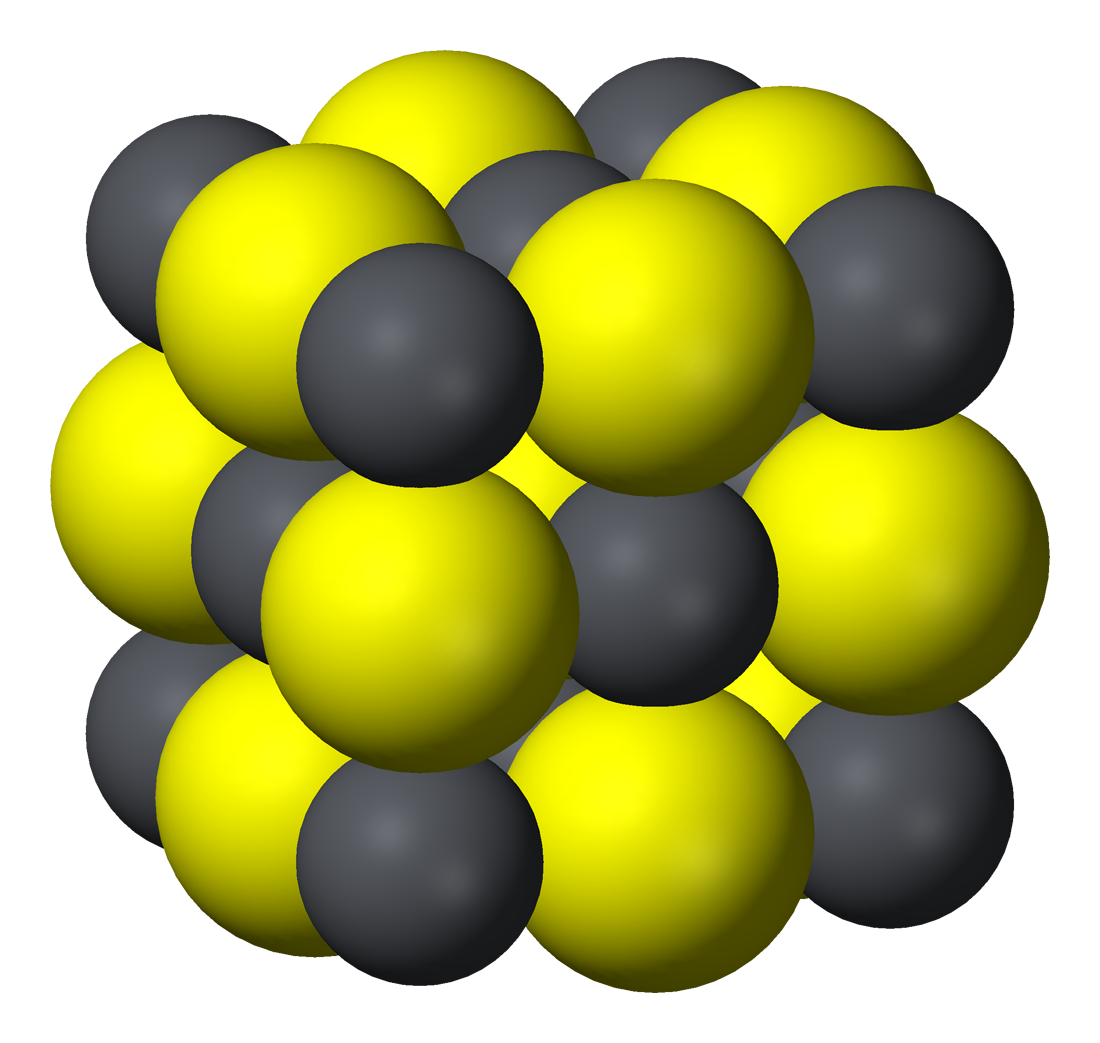
Lead sulphide salt. K sp is the product ot the concentrations of the salt constituent ions each raised to its stoichiometric coefficient. The main structure is the Salt Creek Shear which trends north-south and deforms the belt on a regional scale. For confirmation read todays ICG ann of outcrops up to hundred m long of Cu5 and AG1000 gt see Fig 3 of their ann today - but the key issue is that our turn could come at any time.
The treatment or elimination of pollution from unregulated sources. This is done by conducting a series of tests in a systematic manner and using the observations to confirm the absence or presence of specific cations and anions. 89134 anhydrous basis Compare Product No.
B Print A thin strip of type metal used to separate lines of type in printing. And X denotes a halide I Br or Cl Corner-shared PbX 6 octahedra form a cuboctahedral cage to accommodate the A cation that satisfies the steric requirements and. C Sheets or plates of lead used as a covering for roofs.
Empirical Formula Hill Notation. Lead chloride is a crystalline salt whose formation is encouraged by micro holes in the wall larger surface. Take a salt solution and add cobalt nitrate to it.
Since w e are performing a semi. Group Precipitation cold Pb2 2Cl- PbCl 2 in group l Pb2 S2-H PbS. Galena is the principal ore and the most important compound of lead.
We must also ensure a quantitative precipitation. So we may not get the required clear original solution. An article made of lead or an alloy of lead.
Salt analysis is an integral part of the CBSE Class 12 Chemistry. Its preparation is an entertaining and popular. Purple or violet a Take 01 g of salt in a china dish.
Is iron sulphide compounds. 90 HPLC Expand. The corrosion data in this section is mainly based on the results of general corrosion laboratory tests which are not strictly comparable with actual service conditionsThe corrosion tables provide an initial guide to the selection of materials and are intended to facilitate understanding of the different types of corrosion damage that can arise due to poor material selection.
Adult intake of kitchen salt is on average 9 g per. Restacked two-dimensional hexagonal-MoO3 exhibits high pseudocapacitive. Add 1 mL of ethanol and 02 mL conc.
Formation basic properties related materials. No because sulphuric acid H 2 SO 4 will produce sulphates of the cations and some sulphates like lead sulphate PbSO 4 are insoluble. Salt analysis also known as systematic qualitative analysis or qualitative inorganic analysis involves the identification of the cation and anion of an inorganic salt.
At room temperature it is a bright yellow odorless crystalline solid that becomes orange and red when heated. Purple or violet a Take 01 g of salt in a china dish. A A plummet or mass of lead used in sounding at sea.
All could be lost but if Ayra gives us a long intercept of copper or silver lead both strong in the Mt Isa area re Mt Isa itself and Cannington we are off to the bank with a fist full of dollars. 1 Copper oxide and ferrous sulphide 2 Copper sulphide and ferrous oxide 3 Copper sulphide and ferrous sulphide 4 Copper oxide and ferrous oxide 62. Also add a small amount of lead acetateaq to this.
Sodium shortages may lead to dehydration convulsion muscle paralysis decreased growth and general numbness. Hydrogen sulfide appears as a colorless gas having a strong odor of rotten eggs. In the extraction of lead by self reduction.
The planar growth is hypothesized to occur via a match between the crystal lattices of the salt and the growing oxide. Methylmalonyl coenzyme A tetralithium salt hydrate. Explain how Aluminium III Al 3 can be confirmed using cobalt nitrate.
C 25 H 36 Li 4 N 7 O 19 P 3 S xH 2 O. The lead halide perovskite structure is represented by the general chemical formula APbX 3 where A denotes an organic ammonium or inorganic cation such as methylammonium MA formamidinium FA or Cs. Crop fertilization manure application road deicing salt storage areas unlicensed and closed landfills leaks spills and decommissioning clean-up.
It was formerly called plumbous iodide. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Add 1 mL of ethanol and 02 mL conc.
Lead sulphide Black precipitate b If the salt is soluble in water take the solution of salt in water make it alkaline with ammonium hydroxide and add sodium nitroprusside solution. LeadII iodide or lead iodide is a salt with the formula PbI 2. Inorganic minerals metals and compounds such as sodium copper lead and zinc are common in wastewater from both sewage and wastewater.
If it is insoluble in water take sodium carbonate extract and add a few drops of sodium nitroprusside solution. A purple or violet solution is obtained. Generally humans require about 300 mg sodium chloride per day to warrant a balanced sodium level.
MM-CoA α-Methylmalonyl coenzyme A tetralithium salt. The registry of restriction intentions until outcome lists the intentions and Annex XV restriction proposals received by ECHA. They include non-point source activities ie.
It is chiefly obtained from the mineral galena lead sulphide. Density liquid 83 lb gal. The compound currently has a few specialized applications such as the manufacture of solar cells and X-ray and gamma-ray detectors.
Cross-cutting northeast and northwest faults are secondary with Riedal structures occurring between parallel faults. Contact with the unconfined liquid can cause frostbite by evaporative cooling. 1 Smelting 2 Aluminothermy 3 hydrothermy 4 no specific name 63.
LeadII sulfide also spelled sulphide is an inorganic compound with the formula Pb S. Ag Cl - 10-10 K sp When Ag Cl - 10-10 we get a precipitate. Most inorganic substances are stable and cannot be broken down easily by organisms in wastewater.
It is a semiconducting material with niche uses. LeadII ion Pb2 its coordination number is 3 or 4 its oxide is amphoteric dissolves in both acid and alkali Reactions Important in the Separation and identification of Lead Pb2 1. A chemical substance consisting of two atoms of oxygen combined with one atom of another.
Managing director Brett Wallace said drilling would allow the company to target quartzsulphide veinhosted coppergold mineralisation. The process in which metal oxide is reduced to metal by Al is called. They can originate from industrial and commercial sources stormwater and inflow and infiltration from cracked pipes.
Hence pl a roof covered with lead sheets or terne plates. A black precipitate is formed. Lead sulphide Black precipitate b If the salt is soluble in water take the solution of salt in water make it alkaline with ammonium hydroxide and add sodium nitroprusside solution.
Burn a filter paper which. A restriction proposal may be prepared by a Member State or by ECHA at the request of the Commission or on its own initiative for substances in the Authorisation List. H 2 SO.
Multi-media considerations may lead to more stringent limits compared to those needed to protect the water resource alone. People that have diarrhoea or other health effects that increase salt requirements need a higher dietary amount of sodium than usual. Addition of hydrogen sulfide or sulfide salts to a solution containing a lead salt such as PbCl 2 gives a black precipitate of lead sulfide.
13 Copper Production and Recycling. The CuSO 4 molecule consists of an ionic bond between the copper cation Cu 2 and the sulfate.

Neutralisation Reactions Acid Base Salt Water Acids And Bases Are Chemically The Opposite Of Each Other Mixed Together They Cancel Each Other Out Ppt Download
Red copperI oxide then precipitates out of the reaction mixture which indicates a positive result ie.

Is copper oxide a basic salt. Oxide itself is the dianion of oxygen an O 2 molecular ion. Alternatively the copper oxide may also directly react with CO 2 H 2 O and sulfur oxides to. A continuous production process top vs.
16 Reverberatory Melting Furnace. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. That redox has taken place this is the same positive result as with Benedicts solution.
12 Copper Production and Recycling. Hawleys Condensed Chemical Dictionary. The liquid is a preparation of eugenol which reacts with the powder to form an amorphous chelate of zinc eugenolate.
Color in Rock Salt. The blue color of the solution indicates that excess copperII sulfate remains after all of the sodium sulfide has reacted. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
Boiling point - the temperature at which a liquid turns into a gas. The basic reaction of copper and atmospheric oxygen which converts copper to copper oxide is. 2 sodium nitrate nitric acid or sodium dichromate is added.
When 5 mL of 01 M copperII sulfate is mixed with 1 mL of 01 M sodium sulfide the solution becomes pale blue and a black precipitate forms. The oxides that can form during the oxidation process of copper to patina include. Zinc oxideeugenol cement Fig.
Byproduct of copper electrolysis and etching process. Chemical or scientific names are used to give an accurate description of a substances composition. Even so you rarely ask someone to pass the sodium chloride at the dinner table.
17 Electric Arc Melting Furnace Types. However it can be noted that the anhydrous form of this salt is a powder that is white. It is important to note that Fehlings will not work with aromatic aldehydes.
John Wiley Sons Inc. The ICSC project is a common undertaking between the World Health Organization WHO and. The zinc oxideeugenol cements are used to provide a sedative.
Most of the Earths crust consists of solid oxides the result of elements being oxidized by the. Hazardous Substances Data Bank HSDB Copper sulphuric acid salt formation. There is a strong demand and market for sulphuric acid in the DRC to recover copper from oxide ores.
Zinc both in elemental or in its salt forms has been used as a therapeutic modality for centuries. Long-term exposure in rats and mice showed no overt signs of toxicity other than a dose-related reduction in growth. Its use has expanded manifold over the years for a number of dermatological conditions including infections leishmaniasis.
Are all precipitates insoluble in water. 2 Cus O 2 g -- 2 CuOs The copper to patina conversion is a multiple-step process and it takes a long time to form copper carbonate. Blue color sometimes results from defects in the salts crystal structure known as color centers.
Sulfide ores Bornite chalcocite and chalcopyrite oxide ores Malachite azurite and chrysocolla. 212 contains zinc oxide rosin and zinc acetate in the powder. Copper mines in the DRC currently import significant volumes of sulphur used in sulphur-burning acid plants to produce sulphuric acid for the treatment.
Red pink or brown color in salt can be caused by iron oxide stains or trace elements. Copper Production and Recycling. Topical preparations like zinc oxide calamine or zinc pyrithione have been in use as photoprotecting soothing agents or as active ingredient of antidandruff shampoos.
Copper Production and Recycling. An oxide ˈ ɒ k s aɪ d is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. Copper oxide reacts with this element to form blue-colored copper sulfate which may further react with carbon dioxide and moisture present in air to form the patina layer.
Two basic types of copper ores. The name most commonly refers to the compound with formula Cu 2 CO 3 OH 2It is a green crystalline solid that occurs in nature as the mineral malachite. Precipitation Reactions The precipitate forms because the solid AgCl is insoluble in.
Pickling to remove the very tenacious oxide formed during an oxidising anneal or hot working is carried out with a pickling solution of hot 15 sulphuric acid to which approx. Copper mines in the DRC currently import significant volumes of. A negative result is the absence of the red precipitate.
Lewis RJ Sr Ed. Melting point - the temperature at which a solid turns into a liquid. Basic copper carbonate is a chemical compound more properly called copperII carbonate hydroxideIt is an ionic compound a salt consisting of the ions copperII Cu 2 carbonate CO 2 3 and hydroxide OH.
The major soluble salts copperII sulfate copperII chloride are generally more toxic than the less soluble salts copperII hydroxide copper II oxide. The main target users are workers and those responsible for occupational safety and health. This form is characterized by its bright blue colour.
The rosin increases fracture resistance and the zinc acetate is effective in accelerating the reaction rate. Its important to remember that common names are inaccurate and vary from one place and time to another. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
In this case Tollens reagent should be used. Action of dilute sulfuric acid on copper or copper oxide often as oxide ores in large quantities with evaporation and crystalization. A specimen of rock salt from Bischofferode Thuringia Germany.
Pickling treatments can be avoided by quenching from elevated temperatures into. The most common form of copper sulfate is its pentahydrate given by the chemical formula CuSO 45H 2 O. Death is preceded by gastric hemorrhage tachycardia hypotension hemolytic crisis convulsions and paralysis.
Warm hydrochloric acid 11 with a bichromate addition is also suitable. Metal oxides thus typically contain an anion of oxygen in the oxidation state of 2. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
Copper is known as a noble metal which provides appropriate corrosion resistance in the atmosphere and in some of chemical environments due to the formation of a protective passive oxide film or nonconductive layer of corrosion products on its.