Density of aqueous solutions of some inorganic substances - Changes in density of aqueous solutions with changes in concentration at 20C. 1 mol of ethanol C 2 H 5 OH 1 mol of formic acid HCO 2 H or 1 mol of water H 2 O.
Answered Formic Acid Is Partly Responsible For Bartleby
Density of acetic acid citric acid formic acid D-lactic acid oxalic acid and trichloroacetic acid in water is plotted as function of wt molkg water and moll solution.

Molar mass of formic acid. How are the molecular mass and the molar mass of a compound similar and how. For 20 ml acid solution. Which contains the greatest number of moles of oxygen atoms.
1 00000175 00000175 M. To learn the technique of titration and apply it to determine the molar mass of an unknown weak acid by titration with sodium hydroxide. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
Among them hydrogen mass density and volume capacity of formic acid FA are as high as 44 wt and 534 gL 1 respectively. Therefore 060 mol of formic acid would be equivalent to 120 mol of a compound. Which contains the greatest mass of oxygen.
So in 20 ml of acidic solution 180 x 10-3 equivalent of acids. PH is logarithmically and inversely related to the concentration of hydrogen ions in a solution. Product identifier used on the label.
075 mol of ethanol C 2 H 5 OH 060 mol of formic acid HCO 2 H or 10 mol of water H 2 O. A Calculate the concentration of the unknown acid solution. Chemical formula C 2 H 4 O 3 also written as HOCH 2 CO 2 H is the smallest α-hydroxy acid AHA.
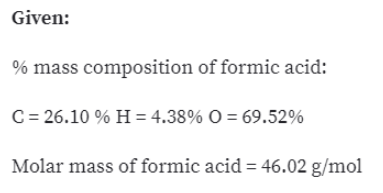
075 mol of ethanol C 2 H 5 OH 060 mol of formic acid HCO 2 H or 10 mol of water H 2 O. The molar mass of formic acid is equal to 46025 grams per mole. Which contains the greatest mass of oxygen.
Which contains the greatest number of moles of oxygen atoms. Which contains the greatest mass of oxygen. It is known to be the active component of vinegar which is a 4 7 solution of acetic acid in water.
Recommended use of the chemical and restriction on use. Its formula has twice as many oxygen atoms as the other two compounds one each. Glycolic acid is found in some sugar-crops.
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Hydroxides are strong bases but have low An acid dissociation constant K a is a quantitative measure of the strength of an acid in solution. Formic acid although not widely used as a solvent is of interest as an example of a protic solvent with high acidity.
The 42-Da mass shift due to acetylation is shown in Figure 63. 075 mol of ethanol C 2 H 5 OH 060 mol of formic acid HCO 2 H or 10 mol of water H 2 O. Acid anhydride Acid catalyzed Acid chloride.
PH -log1H Where. Glycolic acid hydroxyacetic acid or hydroacetic acid. For weak acid such as acetic acid its pKa00000175 thus H concentration of 1 M acetic acid is.
Formylation from formic acid can also occur as an artifact during protein purification if formic acid is used. 1 mol of ethanol C 2 H 5 OH 1 mol of formic acid HCO 2 H or 1 mol of water H 2 O. Which contains the greatest mass of oxygen.
4551 mL of a 01011 M NaOH solution is used to neutralize 5000 mL of an unknown acid. 15 ml 012 mol NaOH required. Three 250-mL Erlenmeyer flasks one 250mL beaker a sample bottle - mL 50-buret 0100 M NaOH solution standardized sodium hydroxide phenolphthalein indicator pH meters.
Therefore x 9 10-3 equivalent because it is a monobasic acid the mass of the titration equation of the acid is the same. The resins is then acidified to pH 4 with formic acid and reacted for a further 520 min. Acetic acid also known as ethanoic acid is a weak acid with the chemical formula CH 3 COOH.
Formic Acid CH3COOH Acetic Acid CH4 Methane CH4O Methanol CHCl3 Chloroform Cl2 Chlorine Gas ClO2 Chlorine Dioxide CoNO32 CobaltII Nitrite CO2 Carbon Dioxide CoCl2 CobaltII Chloride COCl2 Phosgene CoSO4 CobaltII Sulfate CrOH3 Chromium Hydroxide Cr2O3 ChromiumIII Oxide CrCl3 ChromiumIII Chloride CS2 Carbon Disulfide CsCl Caesium. How are the molecular mass and the molar mass of a compound similar and how. Acetic acid is a weak acid because it only partially dissociates into its constituent ions when dissolved in water.
The resulting resin is then stabilized by neutralizing to a pH 75. To prepare a suitable resin formalin is first neutralized. The mixture is boiled under reflux typically for about 15 min to give dimethylol urea and other low molar mass products.
Therefore 060 mol of formic acid would be equivalent to 120 mol of a compound containing a single oxygen atom. Formic acid 85. This colorless odorless and hygroscopic crystalline solid is highly soluble in waterIt is used in various skin-care products.
075 mol of ethanol C 2 H 5 OH 060 mol of formic acid HCO 2 H or 10 mol of water H 2 O. The reaction of sulfuric acid H 2 SO 4 with Sodium. Hydrogen ion concentration in the solution H concentration of acid is depended on its pKa for strong acid like HCl its pKa1 thus H concentration of 1 M HCl is also 1 M.
It is a non-toxic harmless and cheap chemical hydrogen storage material that can effectively avoid the problem of hydrogen storage and transportation 89. A glycolate or glycollate is a salt or ester of glycolic acid. Determining Molar Mass of an Unknown Acid by Titration.
So the number of base equivalents 12 15 18 10-3 equivalent. The pH scale pH is a numeric scale which is used to define how acidic or basic an aqueous solution is. Formic acid 85.
The pH to H formula that represents this relation is. Formic acid is a colorless fuming liquid with a pungent acrid odor with the chemical formula HCOOH. What is Formic Acid.
Formic acid is systematically named as methanoic acidThe common names for simple carboxylic acids come from the Latin or Greek names of their source. In the corresponding MSMS spectra all of the fragment ions containing the acetyl group are also shifted by 42 Da. It commonly ranges between 0 and 14 but can go beyond these values if sufficiently acidicbasic.
Its formula has twice as many oxygen atoms as the other two compounds one each. Moles gramsmolar mass. Industrial chemicals The Recommended use identified for this product is provided solely to comply with a Federal requirement and is not part of the sellers published specification.
Molar mass Mole Molecular formula. Formic acid systematically named methanoic acid is the simplest carboxylic acid and has the chemical formula H 2 CO 2It is an important intermediate in chemical synthesis and occurs naturally most notably in some antsThe word formic comes from the Latin word for ant formica referring to its early isolation by the distillation of ant bodies. 00 M perchloric acid HClO 4 Jun 23 2021 The molar mass of any element can be determined by finding the atomic mass of the element on the periodic table.
Formic acid Formyl group. Ka of formic acid 18 x 10-4 View Answer.
But when a wasp stings a person it. The chemical formula of formic acid is rmHCOOH.

Table Of Alkanes With Formula Structural Formula And State At Room Chemistry Lessons Structural Formula Chemistry Worksheets
Uses of Formic Acid.

Chemical formula for formic acid. Dry chemical carbon dioxide water spray or alcohol resistant foam. 94 rows Formula CH2O2 SMILES OCO Molecular Weight 1 4603 CAS 64-18-6 Other Names. A chemical formula of formic acid can therefore be written as.
The chemical or molecular formula for Formic Acid is HCOOH or HCO 2 H. To meet the multiple specifications and requirements of our respected clientèle we also alter the packed quantities of these products. Formic acid for synthesis.
Formic acid also methane acid an organic substance that belongs to the saturated monobasic carboxylic acids. The common names for simple carboxylic acids come from the Latin or Greek names of their source. Flash Point and explosive limits are for 90 aqueous solutions of formic acid.
It consists of a carboxyl group along with an aldehyde group. Formic acid Formula Formic acid is the simplest carboxylic acid also called methanoic acid. CH2O2 The chemical formula of formic acid shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information which is different from the empirical formula which provides the numerical proportions of atoms of each type.
2Baking soda is applied on the stung area because rubbing a mild base like baking soda solution on the stung area gives relief as it neutralises the acidic liquid injected by the bee. What is the chemical formula for formic acid. - Find MSDS or SDS a COA data sheets and more information.
One of the most common industrial uses. The chemical formula of formic acid CH ₂O co₂. Formic acid is a colorless fuming liquid with a pungent acrid odor with the chemical formula HCOOH.
The chemical formula of formic acid is HCOOH or HCO2H. HCOONa H 2 SO 4 HCOOH NaHSO 4 Methyl Alcohol. Above flash point vapor-air mixtures are explosive within flammable limits noted above.
Formic acid systematically named methanoic acidThe chemical composition is HCOOH. 1The chemical formula of formic acid is CH2O2. It is a colourless liquid with a pungent odour and has a sour taste.
CAS 64-18-6 EC Number 200-579-1 chemical formula HCOOH. Formic acid has the chemical formula of HCOOH or CH2O2 where a hydrogen atom is attached to the -COOH group to form the simplest carboxylic acid. Do you know that formic acid can naturally occur in several species of.
Carbonous acid Formylic acid Hydrogen carboxylic acid Hydroxyoxomethane Metacarbonoic acid Methanoic acid Oxocarbinic acid Oxomethanol INCI. The rational formula of formic acid HCOOH. Soluble in alcohol ether and water.
Formic acid HCOOH or CH2O2 CID 284 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. Formic acid is obtained by adding appropriate amounts of sulfuric acid to the sodium format. Sensitive to static discharge.
COMMON NAME SYSTEMATIC NAME CAS RN FORMULA MELTING POINT Formic Acid Methanoic acid 64-18-6 HCOOH 85 C Acetic Acid Ethanoic acid 64-19-7 CH 3COOH 165 C Carboxyethane Propionic Acid 79-09-4 CH 3CH 2COOH -215 C Butyric Acid n-Butanoic acid 107-92-6 CH 3CH 22COOH -8 C. It is also known as methanoic acid. It is corrosive and is miscible in acetone methanol ether ethyl acetate ethanol and ether.
Formic acid is systematically named as methanoic acid. We are known as one of the reputed manufacturer and supplier of a wide array of Formic Acid. Formic Acid Chemical Formula.
Formic acid is obtained by oxidation of methyl alcohol. Fire data listed is for formic acid.
In the presence of platinum formic acid decomposes with a release of hydrogen and carbon dioxide. Its molecular formula is CH 2 O 2 and its molar mass is 4602 gmol.

Naming Alkanes 20 Multiple Choice Question For New A Level Inc Edpuzzle Link Teaching Resources Choice Questions Multiple Choice Answering Multiple Choice Questions
This C-1 compound is formed in equimolar ratio in comparison to levulinic acid.

Formic acid structural formula. Formic acid is completely soluble in water. The chemical formula of formic acid is HCO2H or HCOOH. A The bond order of C-O is one and C O is two.
HCOOH is known as formic acid common name or methanoic acid IUPAC name. In this formula the two hydrogens are not written combined as H2CO2 to distinguish the. More the bond order more is the.
Formic acid HCO 2 H also called methanoic acid the simplest of the carboxylic acids used in processing textiles and leather. This problem has been solved. Rank the relative strengths of a the CO and CO bonds and b the HC and HO bonds.
Carboxylic acids are organic compounds with RCOOH formula where R can be H First member or alkyl group higher members. The chemical formula of formic acid is HCOOH or HCO2H. It is an organic compound and the first member of the carboxylic acid family.
Chemical name CAS formic acid CAS number 64-18-6 Structural formula HCOOH Molecular formula CH 2O 2 Molecular weight 4603 Melting point 8C Boiling point 101C Density at 20C 122 gcm3 Vapour pressure at 20C 42 hPa log P ow 054 1 mlm3 ppm 191 mgm3 1mgm3 0524 mlm3 ppm. Sodium hydroxide NaOH is now added and the pH. The general structural formula of the carboxylic acid is R C O O H where R stands for any side group comprising H or an alkyl group or another C bonded to a certain chain.
To learn more about the structurePhysical properties chemical properties uses and FAQs visit BYJUS for more information. Formic Acid HCOOH- Formic acid is the simplest carboxylic acid with the chemical formula HCOOH. It is a colorless fuming liquid with a pungent odor.
Formic acid systematically named methanoic acid is the simplest carboxylic acid and has the chemical formula H₂CO₂. The Lewis structure of formic acid is given below showing the lone-pair of electrons on. It is a colourless liquid with a pungent odour and has a sour taste.
Hybridisation of the carboxylic carbon atom Number of atoms attached. Currently the use of formic acid in fuel cells is under investigation 51. It is an important intermediate in chemical synthesis and occurs naturally most notably in.
Formic acid is the simplest carboxylic acid. Formic acid is a by-product of the production of levulinic acid from hydroxymethylfurfural. It is an important intermediate in chemical synthesis and.
Formic acid is obtained by adding appropriate amounts of sulfuric acid to the sodium format. HCOOH Lewis Structure Molecular Geometry Hybridization and Polarity. The chemical or molecular formula for Formic Acid is HCOOH or HCO 2 H.
The structural formula of formic acid is given below. HCOONa H 2 SO 4 HCOOH NaHSO 4 Methyl Alcohol. Formic acid is the simplest carboxylic acid.
Formic acid is obtained by oxidation of methyl alcohol. The general structural formula of the carboxylic acid is rm RCOOH where rm R stands for any side group comprising rm H or an alkyl group or another rm C bonded to a certain chain. It is corrosive and is miscible in acetone methanol ether ethyl acetate ethanol and ether.
3 0 3. Formic acid HCOOH or CH2O2 CID 284 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. Formic acid was first isolated from certain ants and was named after the Latin formica meaning ant.
Hybridisation of the carboxylic carbon atom Number of atoms attached Lone pairs. Formic acid systematically named methanoic acid is the simplest carboxylic acid and has the chemical formula HCOOH. Formic acid is the simplest carboxylic acid also called methanoic acid.
See full answer below. Structural formula shown below is secreted by certain species of ants when they bite.