Total number of protons in the cell nucleus is referred to as the atomic number of the atom and is offered the symbol Z. Today there are many calculators for converting one value to another and vice versa.

Physical And Chemical Properties And Changes Worksheet Together With Do Now Tuesda Physical And Chemical Properties Fraction Word Problems Rational Expressions
16 neutrons Phosphorus has 16 neutrons.

How many protons in phosphorus. A charge of -3 indicates that it has gained 3 electrons. Phosphorus atomic number is 15. How Many Protons Does Phosphorus Have.
How many neutrons are present in phosphorus. It is provided in life-scientific research laboratories in applications in which lower energy beta egoals are beneficial such as DNA sequencing. Phosphorus is used in the red tips of ordinary kitchen matches.
Suvijakra Shutterstock You are watching. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Phosphorus has 16 neutrons.
The number of protons and electrons in a neutral atom of the Jorge baked cookies for his math classs end of. Does phosphorus have 17 neutrons. Phosphorus 15 protons 16 neutrons and 15 electrons Level 1 2 electrons Level 2 8 elec Level 3 5 elec.
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. How many protons neutrons and electrons are in an atom of phosphorus 31. It has 17 protons 17 electrons and 18 neutrons 35 protons 35 electrons and 17 neutrons.
To find the number of neutrons subtract the atomic from the mass. Phosphorus is a chemical element with atomic number 15 which means there are 15 protons in its nucleusTotal number of protons in the nucleus is called the atomic number of the atom and is given the symbol ZThe total electrical charge of the nucleus is therefore Ze where e elementary charge equals to 1602 x 10-19. 33P a beta-emitter 025 MeV through a half-life of 254 days.
Similarly you may ask how many electrons and neutrons does phosphorus have. How Many Protons Neutrons And Electrons Are There In Phosphorus. That is phosphorus P atoms have a total of fifteen electrons.
The atomic number is the number of protons. Phosphorus has 16 neutrons Phosphorous is 15 on the periodic table which means that the atomic number number of protons of phosphorous is 15. How many protons neutrons and electrons are in an atom of phosphorus.
Phosphorus is a chemical facet with atomic number 15 which means there space 15 protons in the nucleus. That is the number of protons in the phosphorus P is fifteen. This isotope is phosphorus 31 which has atomic number 15 18 feb 2016 of course the protons z determines identity.
Phosphorus is a chemical facet with atomic number 15 which way there are 15 protons in that nucleus. How do I know how many protons does phosphorus have. An element has an atomic number of 29.
The chemical symbol for Hydrogen is H. To do this you need to write in the search box for example google how many protons does phosphorus have and add to it an additional word. Phosphorous is 15 on the periodic table which means that the atomic number number of protons of phosphorous is 15.
How many protons neutrons and electrons make up an ion of phosphorus-28 that has a -3 charge. Atomic Number Protons Electrons and Neutrons in Phosphorus. Phosphorus the 15th element on the periodic table was first distilled by an alchemist searching for gold searching that is in at least 60.
31P is phosphorus only stable isotope with 31 - 15 16 neutrons. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Phosphorus-33 is written of 15 protons 18 neutrons and also 15 electrons.
Now the phosphide ion P3- has a total of 18 electrons without any change in its numbers of protons and neutrons in its nucleus. How many electrons does p3- have. Phosphorus atoms will gain three electrons to.
What element has a mass number of 31 and 15 neutrons. Phosphorus 32 P-32 is the phosphorus isotope whose nucleus consists of 15 protons and 17 neutrons. Electrons equal to protons are located in a circular shell outside the nucleus.
Total number of protons in the cell nucleus is called the atomic number of the atom and also is provided the symbol Z. Making its total electrons 18. Protons and Neutrons in Phosphorus.
Therefore it has 18 electronsTable 41Maximum electrons18. Therefore it has 15 protons. Phosphorus is a non-metallic element in the p-block on the periodic table.
This is the isotope phosphorus-31. At the touch of a button you can find out how many protons does phosphorus have. Phosphorous is 15 on the periodic table which means that the atomic number number of protons of phosphorous is 15.
31-1516 neutrons inside the nucleusPhosphorus 15 protons 16 neutrons and 15 electrons Level 1 2 electrons Level 2 8 elec Level 3 5 elec. Phosphorus Atomic Mass Atomic Weight P. An interesting feature in terms of electron configuration EC is that the nearest noble gas atom to Phosphorus Argon Ar with atomic number 18 also has an EC of 288.
It has 15 protons 15 electrons and 16 neutrons. See full answer below. Its atomic number is 15 and its atomic mass is 30974 gmole.
P Phosphorus has an atomic number of 15. How many protons does phosphorus have. Thus neutral phosphorus has 15 protons and 15 electrons.
Phosphorus is a chemical element with atomic number 15 which means there are 15 protons in its nucleus. Phosphorus-33 atomic number 15 contains 18 neutrons 18 protons.
Isomers of c5h11cl. Instead the number and structural organization of carbons is a definitive characteristic.
Name this root chain using the alkane rules.
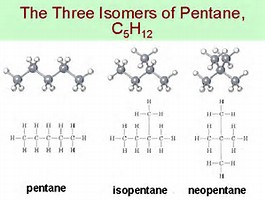
How many carbons are in isopentane. The final gasoline product produced by the refining operation is a mixture of aromatic and aliphatic organic compounds with the number of carbons ranging from C 4 to C 12 2. Isopentane or 2-methylbutane Shortening the main chain to four C-atoms and looking for positions to place one methyl group Neopentane or 22-dimethylpropane Main chain has three carbons in this case So just keep on shortening the main chain and look for the positions where you can place your methyl sometimes even ethyl groups. 12 How many distinct chloropentanes C5H11Cl could be produced in the direct chlorination of n-pentane CH3CH23CH3.
Saturated hydrocarbons are the simplest of the hydrocarbon species. For example isopentane neopentane and n-pentane are names of branched forms of the alkane pentane. How many milligrams per liter as.
The names formulas and physical properties for a variety of alkanes with the generic formula C n H 2n2 are given in the table below. The four-carbon alkane is butane with the formula C 4 H 10. So root word is Hex The compound is saturated or otherwise it consists of only single bond.
The compound at the far right discovered after the other two was named neopentane from the Greek neos meaning new. Although all three have the same molecular formula they have different properties including boiling points. Number the root chain so that the side chains have.
Isopentane for one hour. The alkane that contains three carbon atoms is known as propane which has the formula C 3 H 8 and the following skeleton structure. Nihon Hoigaku Zasshi 45 2.
Male mice of ICR strain were fed with 80 mgkg bw. As defined by IUPAC nomenclature of organic chemistry the classifications for hydrocarbons are. Name each side chain according to its number of carbons but change the suffix of its name from -ane to -yl.
The prefix will be placed in front of the alkane name that indicates the total number of carbons. This resulted in an increase in the amount of. One of the isomers is an effective medication the other caused the side effects.
Structural Isomers Just how many structures can you make from a simple formula. Answer 1 of 2. Because of this terpenes usually have 5n carbon atoms n is an integer and are subdivided as follows.
If you go on constructing structures for higher alkanes you will be getting still larger number of isomers. The naming rules are somewhat complicated. The formula for acyclic saturated hydrocarbons ie alkanes is C n H 2n2.
Isomers of c5h11 Isomers of c5h11oh. Primary a hydrogen on. For example the boiling points for n-pentane isopentane and neopentane at 1 atm are 361C 278C and 95C respectively.
They are composed entirely of single bonds and are saturated with hydrogen. As many as 75 isomers are possible for C10H22. Thus neopentanes lower boiling point means that this isomer has a lower vapor pressure which makes it is more likely to enter the atmosphere than the other two isomers under the same environmental conditions.
67 estimate 2. Blending operations ensure that the gasoline is within specifications. Indeed proton transition energy under dDNP conditions lies within the EPR width.
C6H14 has got five isomers and C7H16 has nine. To assign the prefixes sec- which stands for secondary and tert- for tertiary it is important that we first learn how to classify carbon molecules. 2825 K V 2019-20.
Alkanes have the general chemical formula C n H 2n2The alkanes range in complexity from the simplest case of. Like isobutane it has a one CH 3 branch off the second carbon atom of the continuous chain. In organic chemistry an alkane or paraffin a historical trivial name that also has other meanings is an acyclic saturated hydrocarbonIn other words an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carboncarbon bonds are single.
C2H6 Once the two carbons are connected there are only six additional bonding sites and these are filled by the six hydrogen atoms. 301 K IV 22-Dimethylpropane neopentane bp. Isopentane was metabolized chiefly by liver microsomes.
Very few analytical methods can address so many applications such as. Likewise this you can calculate the structural isomerism of. So the primary suffix is ane.
This class of radicals was used the first time in. However direct carbon polarization remains less efficient than for narrower line radicals. The compound in the middle is isopentane.
What is the major product for the following reaction. CH4 C H H H H CH4 C H H condensed formula 2D formula 3D formula methane 2. The length of the carbon chain is six carbon.
Ethane is a saturated molecule. The boiling points of the alkanes gradually increase with the. Making it suitable for thermal mixing or cross-effect DNP mechanism for both protons and carbons.
In structures II IV and V you observed that CH3 group is. Was the gas pure n-pentane pure isopentane pure neopentane or a mixture of two or all three of these. H2so4 hbr hi i br hso4 tsoh hno3 hf o h o h o h h o h o h h o h o o o h nh h2co3 hn 3 o h h h2s hcl cl h f n no3 sh tso- hco3 n o o-10-9-8-3.
Academiaedu is a platform for academics to share research papers. Isobutane which is the same as 2-methylpropane. The average molecular weight of the final gasoline blend varies from 92 to 95 2.
Find the longest chain of carbon atoms. How many grams of iron oxide Fe2O3 can be produced from 25 g of oxygen reacting with solid iron. Terpenes may be considered to be made up of isoprene more accurately isopentane units an empirical feature known as the isoprene rule.
Chiba S Oshida S. The IUPAC name of CH3-CH2-CH2-CH2-CH2-CH3 is Hexane This name is given as. A refining process which alters the fundamental arrangement of atoms in the molecule without adding or removing anything from the original material.
Academiaedu is a platform for academics to share research papers. 2-methyl-2-butanol in the isopentane inhalation experiment. Pentane shows structural isomerism of three types.
What does iso mean. If you have the problem with calculating isomers of alkane just try to write the structure of the compound that is the easiest way to find out the isomerism of alkane. Of phenobarbital for consecutive four days and exposed to.
An organization the International Organization for Standardization that sets standards in many businesses and technolo. Used to convert normal butane into isobutane C4 an alkylation process feedstock and normal pentane and hexane into isopentane C5 and isohexane C6 high-octane gasoline components. If a carbon is attached to only one.
376 CHEMISTRY to V. Answer 1 of 8. 623 The most general form of saturated hydrocarbons is C n H 2n21.
Isopentane which is the same as 2-methylbutane.
