C 2 H 4 O. Stomach acid is composed of hydrochloric acid HCl potassium chloride KCl and sodium chloride NaCl.
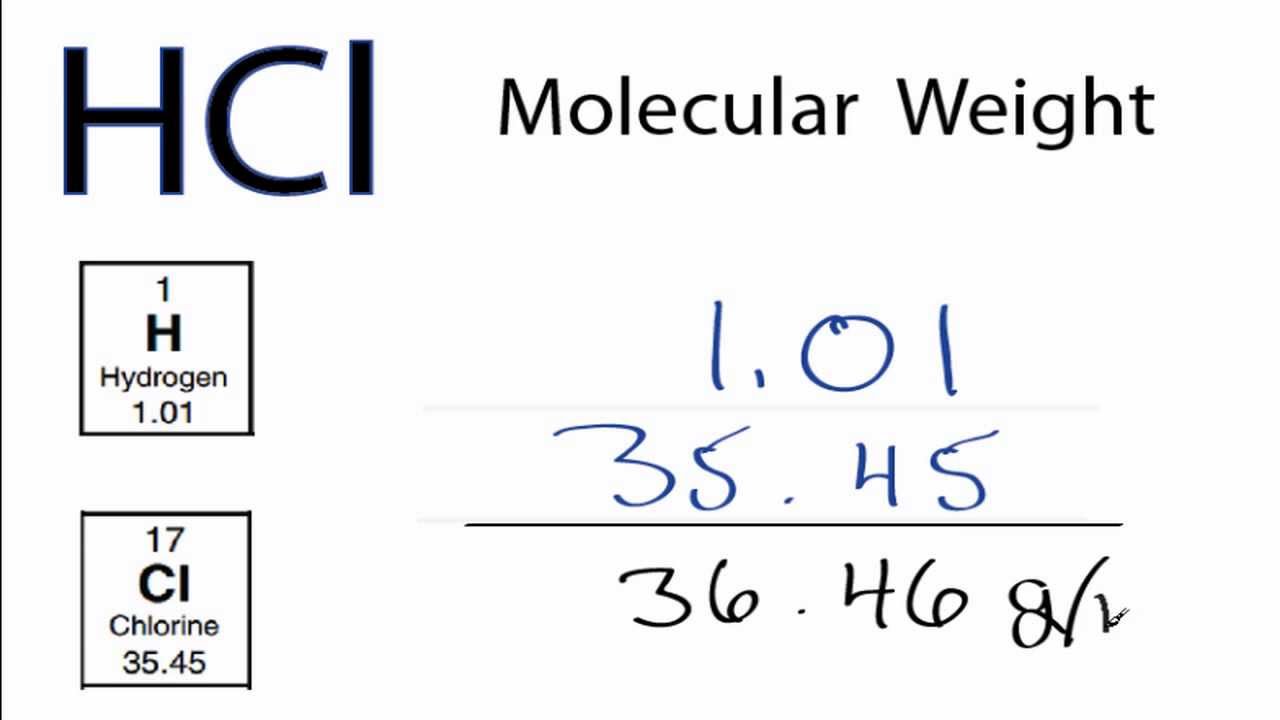
Molar Mass Molecular Weight Of Hcl Hydrochloric Acid Youtube
There are two acid protons in the starting acid sulphuric acid H2SO4.
Hydrobromic acid mass. HC 2 H 3 O 2 aq o H aq C 2 H 3 O 2 aq acetic acid hydrogen ion acetate ion The structural formula for acetic acid is shown to the right. The scale ranges from 2954 to 2771 kcal mol-1 and extends the previously reported scale towards higher acidities. The acid dissociation constant K a is a small value than that of strong acids.
Triflic acid were significantly revised. Although the data on chromic acid is not as comprehensive as data on nitric acid the resistance of titanium to corrosion by chromic acid seems to be quite similar to that seen with nitric acid. Dangerous Goods of Class 8 Packing Group III Solids not listed above.
Chemical compound molar mass. Cole-Parmer provides a free easy to use Chemical Compatability database. 30g 65 g 567 g 78gmol 160gmol 157gmol 30g1mol78g 65g1mol160g.
So the number of base equivalents 12 15 18 10-3 equivalent. So in 20 ml of acidic solution 180 x 10-3 equivalent of acids. It also eliminates stomach bacteria and viruses thereby protecting your body from infection.
Hydrochloric acid concentration in the stomach is. Lets look at HBr for example. Similar calculators pH of a solution calculator Acid-base titration curves Glue solution strength Solution of buckets and tank problem Molarity calculator Chemistry section 19 calculators pH solution acid base.
Hence titanium is resistant to attack in mixtures of strong sulphuric and nitric acids hydrochloric and nitric acids and even. It is an oxoacid of chlorine. It is corrosive to metal and tissue.
The Cativa process is a major end use of hydroiodic acid which serves as a co. Share my calculation Everyone who receives the link will be able to view this calculation. An aqueous solution of calcium hydroxide is standardized by titrated with a 0192 M solution of hydrobromic acid.
Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide HBr in water. There is an increase in. The monoprotic acid is hydrobromic acid because only one proton H can be given.
This study a self-consistent gas-phase acidity scale consisting of 20 superacids was compiled. Properties of Chloric acid. Class 9 Packing Group I Dangerous Goods.
Hydrochloric acid helps the body break down foods such as calcium digest and drink them. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. No monoprotic hydrogen exists.
What is the concentration of a 1mL volume of. Dangerous Goods of Class 8 Packing Group III Liquids not listed above. Replacement methods 7906 7907 7908 allow for the collection of inhalable fractions of acid aerosols by means of a pre-filter and can provide for lower limits of detection for acid gases and vapors due to higher sampling flow rates.
It is a strong oxidizing agent. C 2 H 3 N. For a detailed discussion on preparation and uses of sulphuric acid download BYJUS The Learning App.
Molar mass gmol Density Range of concentration. 001 to 5 mgm. It is a colorless solution.
It has a molar mass of 8445914 g mol 1. That is because the. Hydrazine aqueous solution with more than 37 hydrazine by mass.
Weak acids are molecules that partially dissociate into ions in aqueous solutions. The working range is ca. Titanium provides moderate resistance to reducing acids like sulfuric hydrochloric HCl and phosphoric acid.
It can also be used as a reducing agent for example in the reduction of aromatic nitro compounds to anilines. HBr - Hydrobromic Acid. Hydrobromic acid HBr Hydroiodic acid HI Perchloric acid HClO 4 Chloric acid HClO 3 What is a Weak Acid.
It is usually prepared by two methods the first is by heating the hypochlorous acid that will produce one mole of chloric acid and two moles of hydrogen chloride. If 260 mL of the base is required to neutralize 199 mL of the acid what is the m. Hydrogen is on the left side of the stairs that divide metals and nonmetals in the periodic table but hydrogen is a nonmetal.
Molar mass of naf email protected. 4 HI O 2 2 H 2 O 2 I 2. URL copied to clipboard.
Gas-phase acidities for several important superacids eg. G of benzene reacts with 65 g of bromine and produces 567 g of bromobenzene what is the percent yield of the reaction. Hydrobromic HBr hydrosulfuric H 2 S hydroiodic HI hydrofluoric HF nitric HNO 3 nitrous HNO 2 phosphoric H 3 PO 4 acetic HC 2 H 3 O 2 Only one of the hydrogen atoms of the acetic acid molecule is acidic.
6Br and hydrobromic acid. The pH of the solution is about 3-5. Preparation of Chloric acid.
The hydrogen attached to the. In other words the sulfate molecule was bound to these acidic protons. Constant boiling hydrobromic acid is an aqueous solution that distills at 1243 C and contains 476 HBr by mass which is 877 molL.
An aqueous solution of concentrated hydrobromic acid contains 48 HBr by mass. Carbonic Acid H2O Water H2O2 Hydrogen Peroxide H2S Sulfane H2SO3 Sulfurous Acid H2SO4 Sulfuric Acid H3BO3 Boric Acid H3PO2 Hypophosphorous Acid H3PO3 Phosphorous Acid PhosphoricIII Acid H3PO4 Phosphoric Acid HBr Hydrobromic Acid HCl Hydrogen Chloride HClO4 Perchloric Acid HCN Cyanic Acid HgNO32 MercuryII Nitrate HgCl2 MercuryII. For 20 ml acid solution.
Test your Knowledge on Mass. Hydrobromic acid has a pK a of 9 making it a stronger acid than hydrochloric acid but not as strong as hydroiodic acid. Like other hydrogen halides hydroiodic acid adds to alkenes to give alkyl iodides.
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. 15 ml 012 mol NaOH required. What is the molar concentration of 68 nitric acid for which rho_acid141gmL-1.
Onversion factor to convert from anion to acid concentration c F. On impregnated quartz fiber filters greater than 95 recovery of hydrochloric and nitric acid was found. If the density of the solution is 15 g mL What is its concentration.
C 3 H 6 O. For a 50-L air sample see EVALUATION OF METHOD. C 2 H 5 NO.
While the material normally has a significant corrosion rate in media such as sulphuric or hydrochloric acids which produce hydrogen on reaction with the metal the presence of a small amount of oxidising agent in the acid results in the formation of a passive film. Therefore x 9 10-3 equivalent because it is a monobasic acid the mass of the titration equation of the acid is the same. Our specialists are available to advise you about the best products.
Hydroiodic acid reacts with oxygen in air to give iodine. Weak acids do not release all the H ions to the solution. C 10284 for chloride 10126 for bromide and 10163 for nitrate EVALUATION OF METHOD.