CobaltII chloride ultra dry. Zinc iron II iron III gallium silver lead IV chloride ZnCl 2.
Formula writing rules to write the correct chemical formulas for each compound.

Cobalt ii chloride formula. Iron II chloride 18. Empirical Formula Hill Notation. Copper II Carbonate Formula.
CID 962 Water CID 104730 Cobalt CID 313 Hydrochloric acid Dates. Either chloride ions or ammonia molecules or both remain bonded to the cobalt ion during the reaction and ii the compounds are formulated as shown in Table 91 where the atoms within the square brackets form a single entity which does not dissociate under the reaction conditions. Cobalt chloride CoCl2 MFCD00010938.
E-mail to a friend. Writing the Formula of Complex Ions. Complete these in lab and on your own time for practice.
Copy this to my account. Copper Cu Identify the oxidation state of the central metal ion shown in parantheses. Claims of the formation of tri- and tetrahydrates have not been confirmed.
CobaltII hydroxide CoOH 2 1610 15. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Some nonmetals form a series of polyatomic ions with oxygen all having the same charge.
Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box. Common Cations and Anions Name Formula Charge Name Formula Charge Name Formula Charge aluminum Al 3 3 magnesium Mg 2 2 carbonate CO 3 2 2 ammonium NH 4 1 manganese II Mn 2 2 chlorate ClO 3 1 barium Ba 2 2 manganese III Mn 3 3 chloride Cl 1 cadmium Cd 2 2 mercury I. CobaltII chloride is normally found in the red or pink form.
It can also be formed by reacting with cobaltII chloride with silver nitrate. CobaltII sulfide CoS 410 21. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem.
The -ate forms formula and charge must be memorizedIn some cases the -ate form has three oxygens and in some cases four oxygens. Lead II nitride 37 CoCO 3 cobalt II carbonate 38 CdSO 3 cadmium sulfite 39 CuNO 2 2 copper II nitrite 40 FeHCO 3 2 iron II bicarbonate Name the following chemical compounds. CHEMISTRY 1A NOMENCLATURE WORKSHEET Chemical Formula Nomenclature Practice.
MgOH2 magnesium hydroxide 9. 3 Chemical and Physical Properties Expand this section. Na2CrO4 sodium chromate 19.
CobaltIII hydroxide CoOH 3 1610 44. But note that nitrogen does not follow this pattern ie nitrate NO 3-. 2 Identify the ligands.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. 1 Structures Expand this section. RNR-Marine is a leading dealer for who patterned and manufactured Factory Original-Equipment-Manufacturer OEM canvas for Cobalt 206 226 227 243 246 262 263 273 292 303 323 and 360 boats from 2000 to 2004.
If 1027 g of copperII Chemistry. 2-Special Names Formula Butanol C 4H 9OH Butanoate ion Butyrate ion C 3H 7CO 2-Benzene C 6H 6 Pentanol C 5H 11OH Pentanoate ion-Valerate ion C 4H 9CO 2 Toluene C 6H 5CH 3 Most Common Formula Representations All represent ethanol Example C 2H 6O CH 3CH 2OH Name Molecular Formula Condensed Molecular Formula Structural Formula Line Formula. 83762 free acid basis Compare Product No.
Chemical Formula For Copper. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central.
It can be heated to turn it into the blue form without water. Copper I Chloride Formula. Combined gas law formula.
Degree of Unsaturation Formula. Na2S sodium sulfide 17. Compound Name Type of Compound.
It contains cobalt in its 2 oxidation state. NiS nickel II sulfide Write the chemical formula for each of the following compounds. Density of Gas Formula.
PbCl2 lead II chloride 7. CopperI iodide CuI 1110 12. CopperII arsenate Cu 3 AsO 4 2 7610 36.
Write the balanced chemical equation the overall ionic equation and the net ionic equa- tion for this reaction. CoCl 2 or Cl 2 Co. The combination of aqueous solutions of silver nitrate and sodium chloride is an easy method of synthesizing silver chloride.
It contains cobalt and chloride ions. Cobalt II Nitrate Formula. CobaltII chloride is an inorganic compound of cobalt and chlorine with the formula CoCl 2It is a red crystalline solid.
TrisethylenediaminecobaltIII chloride is an inorganic compound with the formula Coen 3Cl 3 where en is the abbreviation for ethylenediamineIt is the chloride salt of the coordination complex Coen 3 3This trication was important in the history of coordination chemistry because of its stability and its stereochemistryMany different salts have been described. This precipitation is general for the reaction of silver nitrate. CopperII chloride and leadII nitrate react in aqueous solutions by double replacement.
CobaltII chloride also known as cobaltous chloride and cobalt dichloride is a chemical compound. Copy this to my account. KNO3 potassium nitrate 8.
When an aqueous solution of sodium hydroxide NaOH is added to an aqueous solution of chromiumIII nitrate CrNO33 a precipitate of chromiumIII. Copper II Nitrate Formula. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate.
Ionic Compound Naming and Formula Writing List 1. The compound forms several hydrates CoCl 2 n H 2 O for n 1 2 6 and 9. E-mail to a friend.
Write the formula for the complex ion tetraamminecopperII Identify the central metal ion. 2 Names and Identifiers Expand this section. CopperI cyanide CuCN 3210 20.
The names are found by finding the intersection between the cations and anions. LiCIO3 lithium chlorate 10. CopperI chloride CuCl 1210 6.
Cobalt II chloride hexahydrate. Its chemical formula is CoCl 2. Ionic Compounds Naming and Formula Writing.
4 Spectral Information. Chemical formula that corresponds to the name Name Formula 1 NaF 13 potassium fluoride 2 K2CO 3 14 ammonium sulfate 3 MgCl 2 15 magnesium iodide 4 BeOH 2 16 copper II sulfite 5 SrS 17 aluminum phosphate 6 Cu 2S 18 lead II nitrite 7 ZnI 2 19 cobalt II selenide 8 Ca 3PO 42 20 silver cyanide 9 NH 4I 21 copper II bicarbonate 10 MnNO 33 22 iron II oxide 11 FePO. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
Factory Original-Equipment-Manufacturer OEM Canvas for Cobalt boats. Cobalt chloride hexahydrate is a hydrate of cobalt chloride containing cobalt in 2 oxidation state chloride and water moieties in the ratio 126. C 25 H 42 N 7 O 17 P 3 S xLi CAS No.
CopperII carbonate CuCO 3 1410 10. 41 lithium acetate LiC 2 H 3 O 2 42 iron II phosphate Fe 3 PO 4 2 43 titanium II selenide TiSe. The red form has water in it.
Werner proposed the term secondary valence for the. NH42SO4 ammonium sulfate 20. Cobalt II Sulfate Formula.
Hydrogen sulfide can also result from industrial activities such as food processing coke ovens kraft paper mills tanneries and petroleum refineriesHydrogen sulfide is a flammable colorless gas with a characteristic odor of rotten eggs. Chemical Formula Nomenclature Practice.
CobaltII hydroxide CoOH 2 1610 15.
Cobalt sulfide formula. Use the stock form for the transition metals. We had an interest in indium sulfide as a catalyst because S-doped In was shown by Wang and co-workers to. Sodium thiosulfate ____Na2S2O3_____ 3.
CopperI cyanide CuCN 3210 20. Copy this to my account. CopperI chloride CuCl 1210 6.
E-mail to a friend. To further improve its energy density and application high-voltage LiNi 05 Mn 15 O 4 LNMO spinel cathode is highly desirable due to its high energy density low cost environmental friendliness and Co-free nature. CobaltII sulfide CoS 410 21.
Na Mg 2 Non. Show all questions. CopperII arsenate Cu 3 AsO 4 2 7610 36.
KMnO4 potassium permanganate Write the chemical formula for each of the following ionic compounds. Manganese II carbonate MnCO3 73. CopperI iodide CuI 1110 12.
Zinc iron II iron III gallium silver lead IV chloride ZnCl 2. Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution. B The atoms in lithium sulfide are held together by bonds C.
Metals lose electrons to produce positve ions called cations. It is a red crystalline solid. Which statement about lithium sulfide is correct1 point A The chemical formula for lithium sulfide is LiS2.
Potassium bromide KBr 62. Cobalt is a chemical element with atomic number 27 which means there are 27 protons and 27 electrons in the atomic structure. B The atoms in lithium sulfide are held together by bonds C.
The names are found by finding the intersection between the cations and anions. The free element produced by reductive smelting is a hard lustrous silver-grey metal. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Like nickel cobalt is found in the Earths crust only in a chemically combined form save for small deposits found in alloys of natural meteoric iron. Copy this to my account.
8 water molecules octahydrate. Give the formula for the following. Barium sulfide BaS 72.
The chemical symbol for Cobalt is Co. Ionic Compounds Naming and Formula Writing. 13 potassium fluoride is KF 14 ammonium sulfate is NH 42SO 4.
Sodium chloride NaCl and magnesium oxide MgO. The compound forms several hydrates CoCl 2 n H 2 O for n 1 2 6 and 9. Ca2 Calcium S2-Sulfide Sr2 Strontium Se2-Selenide Ba2 Barium Zn2 Zinc Cd2 Cadmium 3 Al3 Aluminum 3- N3- Nitride P3- Phosphide Table 2 Metals That Form More Than One Monatomic Ion ELEMENT ION FORMULA SYSTEMATIC NAME COMMON NAME Chromium Cr2 Chromium II Chromous Cr3 Chromium III Chromic.
Tin IV sulfide 34 TiCN 4 titanium IV cyanide 35 KMnO 4 potassium permanganate 36 Pb 3 N 2 lead II nitride 37 CoCO 3 cobalt II carbonate 38 CdSO 3 cadmium sulfite 39 CuNO 2 2 copper II nitrite 40 FeHCO 3 2 iron II bicarbonate Name the following chemical compounds. Claims of the formation of tri- and tetrahydrates have not been confirmed. Manganese IV sulfide MnS 2 cobalt II bromide CoBr 2 manganese II carbide Mn 2C phosphorus V nitride P 3N 5 gold I iodide AuI nickel III phosphide NiP iron II bromide FeBr 2 copper II sulfide CuS aluminum sulfide Aℓ 2S 3 silicon iodide SiI 4 lead IV carbide PbC aluminum fluoride AℓF 3 arsenic V nitride.
Cobalt is found in the Earths crust only in chemically combined form save for small deposits found in alloys of natural meteoric iron. Barium oxide BaO lithium sulfide Li 2S magnesium bromide MgBr 2. Ionic Compound Naming and Formula Writing List 1.
It is usually supplied as the hexahydrate CoCl 2 6 H 2 O which is. The free element produced by reductive. Which statement about lithium sulfide is correct1 point A The chemical formula for lithium sulfide is LiS2.
The transfer of electrons between metals and non-metals produces charged particles called ions. When the chemical formula for a hydrated ionic compound is written the formula for the ionic compound is separated from the waters of hydration by a centered dot. 2 In a molecule of lithium sulfide there are two atoms of lithium and one atom of sulfur.
An ionic compound is composed of a metal and a non-metal. Complete these in lab and on your own time for practice. Co2SO43 cobalt III sulfate 60.
Synthesis and characterizations of catalysts. NH42S ammonium sulfide K3PO4 potassium phosphate ZnNO32 zinc nitrate Fe2SO43 ironIII sulfate or ferric sulfate CuCO3 copperII carbonate or cupric carbonate Note. In a formula parentheses are used around a polyatomic ion only when there are 2 or more of that polyatomic ion in a formula unit ie when the subscript is not 1.
41 lithium acetate LiC 2 H 3 O 2 42 iron II phosphate. You should complete this by Sunday. Sulfide all-solid-state lithium-ion batteries represent one of the most promising energy storage technologies duo to higher safety and ionic conductivities.
CobaltII carbonate CoCO 3 1410 13. CobaltII chloride is an inorganic compound of cobalt and chlorine with the formula CoCl 2. E-mail to a friend.
Cobalt-based blue pigments cobalt blue have been used since ancient times for. 5 SrS is strontium sulfide 6 Cu 2S is copper I sulfide 7 ZnI 2 is zinc iodide 8 Ca 3PO 42 is calcium phosphate 9 NH 4I is ammonium iodide 10 MnNO 33 is manganese III nitrate 11 FePO 4 is iron III phosphate 12 CoCO 3 is cobalt II carbonate Name to formula problems. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator.
Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound.
2 In a molecule of lithium sulfide there are two atoms of lithium and one atom of sulfur. The dihydrate is purple and hexahydrate is pink. Formula writing rules to write the correct chemical formulas for each compound.
CobaltIII hydroxide CoOH 3 1610 44. Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. Cobalt is a chemical element with the symbol Co and atomic number 27.
Sulfur dioxide ____SO2_____ 2. People can smell it at low levels. It is commonly known as hydrosulfuric acid sewer gas and stink damp.
It was designed by Alfred Stock 1876-1946 a German chemist and first published in 1919. Greek prefixes are attached to the word hydrate to indicate the number of water molecules per formula unit for the compound eg BaOH 2 8H 2 O. Compound Name Type of Compound.
History- The type of naming you will learn about is called the Stock system or Stocks system.
Sodium thiosulfate ____Na2S2O3_____ 3. Formula and data book Chemistry v13.
Cobalt Iii Nitrate Con3o9 Chemspider
Copy this to my account.
Cobalt iii nitrate formula. 83762 free acid basis Compare Product No. K 4FeCN 6 Answer. You should complete this by Sunday.
Aluminum and sulfur. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. The negative ion anion is written second in the name.
Co2SO43 cobalt III sulfate 60. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Ionic Compounds Naming and Formula Writing.
13 potassium fluoride is KF 14 ammonium sulfate is NH 42SO 4 15 magnesium iodide is MgI 2 16 copper II sulfite is CuSO 3 17 aluminum phosphate is AlPO 4 18 lead II nitrite is PbNO 22. Chemical Formula Nomenclature Practice. Cobalt in the complex ion must be 3.
8 water molecules octahydrate. Cobalt III cobaltic Co 3 3 sodium Na 1 hydroxide OH 1 cobalt IV Co 4 4 tin II stannous Sn 2 2 iodate IO 3 1 copper I cuprous Cu 1 tin IV stannic Sn 4 4 iodide I 1 copper II cupric Cu 2 2 titanium II Ti 2 2 nitrate NO 3 1 gold I aurous Au 1 titanium III Ti. 2 of 15 Physical constants and unit conversions Physical constants and unit conversions Absolute zero Atomic mass unit Avogadros constant Ideal gas constant Ionic product constant for water at 298.
Polyatomic ions are ions which consist of more than one atom. Ionic Compounds Naming and Formula Writing. Formula 21 sodium phosphide Na 3 P 22 magnesium nitrate MgNO 3 2 23 lead II sulfite PbSO 3 24 calcium phosphate Ca 3 PO 4 3 25 ammonium sulfate NH 4 2 SO 4 26 silver cyanide AgCN 27 aluminum sulfide Al 2 S 3 28 beryllium chloride BeCl 2 29 copper I arsenide Cu 3.
10 MnNO 33 is manganese III nitrate 11 FePO 4 is iron III phosphate 12 CoCO 3 is cobalt II carbonate Name to formula problems. Write a formula for the ionic compound that forms from each pair of elements. -ite -ito For neutral ligands the common name of the.
Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. E-mail to a friend. E-mail to a friend.
Chromium III Sulfate Formula. Write a formula for the ionic compound that forms from each pair of elements. The metal atom or ion is written before the ligands in the chemical formula.
Chemical Formula For Copper. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound. Again remember that you never have to indicate the number of cations and anions in the name of an ionic compound.
Manganese II carbonate MnCO3 73. Cobalt II Sulfate Formula. The names of some common ligands are listed in Table 1.
Compound Name Type of Compound. Write the chemical formula for each of the following compounds. Formula writing rules to write the correct chemical formulas for each compound.
Potassium carbonate K2CO3 22. TrisethylenediaminecobaltIII chloride is an inorganic compound with the formula Coen 3Cl 3 where en is the abbreviation for ethylenediamineIt is the chloride salt of the coordination complex Coen 3 3This trication was important in the history of coordination chemistry because of its stability and its stereochemistryMany different salts have been described. It was designed by Alfred Stock 1876-1946 a German chemist and first published in 1919.
Sulfate nitrate and -ite eg. Give the formula for the following. Sulfur dioxide ____SO2_____ 2.
Iron III phosphate 66 K 3 N potassium nitride 67 SO 2 sulfur dioxide 68 CuOH copper I hydroxide 69 ZnNO 2 2 zinc nitrite 70 V 2 S 3 vanadium III sulfide Write the formulas for the following chemical compounds. Nirite change the endings as follows. Sodium peroxide Na2O2 63.
3Co cobaltIII ion cobaltic ion Copper Cu copperI ion cuprous ion 2Cu copperII ion cupric ion Gold Au3 goldIII ion Iron Fe2 ironII ion ferrous ion 3Fe ironIII ion ferric ion Manganese Mn2 manganeseII ion manganous ion 3Mn manganeseIII ion manganic ion Mercury Hg 2 2 mercuryI ion mercurous ion 2Hg mercuryII ion mercuric ion Nickel 2Ni nickelII ion Silver Ag. Potassium is the cation and the complex ion is the anion. Sodium nitrite NaNO2 31.
Write a formula for the ionic compound that forms from each pair of elements. Copper II Carbonate Formula. 1 of 15 Formulas Processing of data Chemical reactions reactants products and energy change Aqueous solutions and acidity Chemical equilibrium systems.
His study of chemistry began in Karlsruhe Germany. Greek prefixes are attached to the word hydrate to indicate the number of water molecules per formula unit for the compound eg BaOH 2 8H 2 O. Use the stock form for the transition metals.
Copy this to my account. Copper I Chloride Formula. The names of some common ligands are listed in Table 1.
Complete these in lab and on your own time for practice. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Empirical Formula Hill Notation.
Sodium and sulfur Express your answer as a chemical formula. Combined gas law formula. 71 silicon dioxide SiO 2 72 nickel III sulfide Ni.
For anionic ligands end in -o. When the chemical formula for a hydrated ionic compound is written the formula for the ionic compound is separated from the waters of hydration by a centered dot. HexaamminecobaltIII chloride is the chemical compound with the formula CoNH 3 6Cl 3It is the chloride salt of the coordination complex CoNH 3 6 3 which is considered an archetypal Werner complex named after the pioneer of coordination chemistry Alfred WernerThe cation itself is a metal ammine complex with six ammonia ligands attached to the cobaltIII ion.
Name Formula Charge Name Formula Charge Name Formula Charge. Potassium bromide KBr 62. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central.
C 25 H 42 N 7 O 17 P 3 S xLi CAS No. The positive ion cation is written first in the name. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atoms.
Barium sulfide BaS 72. Chromium VI Oxide Formula. For anions that end in -ideeg.
Cobalt II Nitrate Formula. Strontium and oxygen Express your answer as a chemical formula. Since there are 4 K.
History- The type of naming you will learn about is called the Stock system or Stocks system. Formula Moles of AgCl precipitated per mole of the compounds with excess AgNO3 i PdCl24NH3 2 ii NiCl26H2O 2 iii PtCl42HCl 0 iv CoCl34NH3 1 v PtCl22NH3 0 Example 91 WernerWernerWerner was born on December 12 1866 in Mülhouse a small community in the French province of Alsace. Chromium III Nitrate Formula.
The atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit. KMnO4 potassium permanganate Write the chemical formula for each of the following ionic compounds.
PbCl2 lead II chloride 7. Copper I Chloride Formula.
Cobalt Ii Chloride Hexahydrate H12cl2coo6 Chemspider
The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
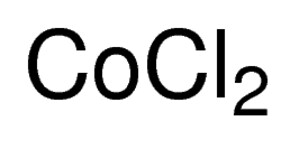
Formula for cobalt ii chloride. CobaltII chloride hexahydrate has a similar structure. The names are found by finding the intersection between the cations and anions. The -ate forms formula and charge must be memorizedIn some cases the -ate form has three oxygens and in some cases four oxygens.
E-mail to a friend. Chemical Formula For Copper. CopperII carbonate CuCO 3 1410 10.
CobaltII sulfide CoS 410 21. The combination of aqueous solutions of silver nitrate and sodium chloride is an easy method of synthesizing silver chloride. Sodium chloride NaCl and magnesium oxide MgO.
The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. Na2CrO4 sodium chromate 19. CoSO 4 or CoO 4 S.
Density of Gas Formula. Complete these in lab and on your own time for practice. The transfer of electrons between metals and non-metals produces charged particles called ions.
LiCIO3 lithium chlorate 10. CopperI cyanide CuCN 3210 20. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate.
CHEMISTRY 1A NOMENCLATURE WORKSHEET Chemical Formula Nomenclature Practice. Either chloride ions or ammonia molecules or both remain bonded to the cobalt ion during the reaction and ii the compounds are formulated as shown in Table 91 where the atoms within the square brackets form a single entity which does not dissociate under the reaction conditions. E-mail to a friend.
CobaltII chloride is normally found in the red or pink form. Copper II Nitrate Formula. It can be heated to turn it into the blue form without water.
Its chemical formula is CoCl 2. Sinks and mixes with water. CobaltII hydroxide CoOH 2 1610 15.
It consists of infinite chains of NiCl 2 wherein both chloride centers are bridging ligands. KNO3 potassium nitrate 8. Na2S sodium sulfide 17.
The compound forms several hydrates CoCl 2 n H 2 O for n 1 2 6 and 9. Copper II Carbonate Formula. C 27 H 42 N 7 Na 2 O 20 P 3 S xH 2 O.
It can also be formed by reacting with cobaltII chloride with silver nitrate. Cobalt II chloride hexahydrate. C 23 H 38 N 7 O 17 P 3 S.
Compound Name Type of Compound. CopperI iodide CuI 1110 12. CopperII chloride and leadII nitrate react in aqueous solutions by double replacement.
Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box. Ionic Compounds Naming and Formula Writing. Sodium sulfate nickel II sulfate iron II sulfate cobalt II chloride magnesium sulfate sodium carbonate calcium sulfate sodium acetate and barium chloride.
Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. CobaltII chloride also known as cobaltous chloride and cobalt dichloride is a chemical compound. Cobalt sulfate is an odorless rose-pink solid.
2 Identify the ligands. CobaltIII hydroxide CoOH 3 1610 44. Iron II chloride 18.
Copy this to my account. MgOH2 magnesium hydroxide 9. Parentheses and a subscript are not used unless more than one of a polyatomic ion is present in the formula unit eg calcium sulfate CaSO 4 not CaSO 4.
The red form has water in it. Cobalt II Sulfate Formula. Empirical Formula Hill Notation.
It contains cobalt and chloride ions. Degree of Unsaturation Formula. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central.
Writing the Formula of Complex Ions. Metals lose electrons to produce positve ions called cations. CobaltII chloride is an inorganic compound of cobalt and chlorine with the formula CoCl 2It is a red crystalline solid.
This precipitation is general for the reaction of silver nitrate. CopperI chloride CuCl 1210 6. Werner proposed the term secondary valence for the.
Claims of the formation of tri- and tetrahydrates have not been confirmed. Chemical formula that corresponds to the name Name Formula 1 NaF 13 potassium fluoride 2 K2CO 3 14 ammonium sulfate 3 MgCl 2 15 magnesium iodide 4 BeOH 2 16 copper II sulfite 5 SrS 17 aluminum phosphate 6 Cu 2S 18 lead II nitrite 7 ZnI 2 19 cobalt II selenide 8 Ca 3PO 42 20 silver cyanide 9 NH 4I 21 copper II bicarbonate 10 MnNO 33 22 iron II oxide 11 FePO. Write the balanced chemical equation the overall ionic equation and the net ionic equa- tion for this reaction.
It contains cobalt in its 2 oxidation state. Na Mg 2 Non-metals gain electrons to produce negative ions called anions. Zinc iron II iron III gallium silver lead IV chloride ZnCl 2.
The dihydrate NiCl 2 2H 2 O adopts a structure intermediate between the hexahydrate and the anhydrous forms. Copy this to my account. 41 lithium acetate LiC 2 H 3 O 2 42 iron II phosphate Fe 3 PO 4 2 43 titanium II selenide TiSe.
Cobalt II Nitrate Formula. Ionic Compound Naming and Formula Writing List 1. When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions.
O is used in photography to fix negatives during the development of photographs. 95562 anhydrous basis Compare Product No. Some nonmetals form a series of polyatomic ions with oxygen all having the same charge.
Empirical Formula Hill Notation. But note that nitrogen does not follow this pattern ie nitrate NO 3-. If 1027 g of copperII Chemistry.
CID 962 Water CID 104730 Cobalt CID 313 Hydrochloric acid Dates. Cobalt chloride hexahydrate is a hydrate of cobalt chloride containing cobalt in 2 oxidation state chloride and water moieties in the ratio 126. CID 1118 Sulfuric acid CID 104730 Cobalt Dates.
Formula writing rules to write the correct chemical formulas for each compound. Five water molecules are attached to every sodium thiosulfate molecule. Write the formula for the complex ion tetraamminecopperII Identify the central metal ion.
The hexahydrate occurs in nature as the very rare mineral nickelbischofite. 2-Special Names Formula Butanol C 4H 9OH Butanoate ion Butyrate ion C 3H 7CO 2-Benzene C 6H 6 Pentanol C 5H 11OH Pentanoate ion-Valerate ion C 4H 9CO 2 Toluene C 6H 5CH 3 Most Common Formula Representations All represent ethanol Example C 2H 6O CH 3CH 2OH Name Molecular Formula Condensed Molecular Formula Structural Formula Line Formula. USCG 1999 CAMEO Chemicals.
Acetyl coenzyme A sodium salt. NiS nickel II sulfide Write the chemical formula for each of the following compounds. NH42SO4 ammonium sulfate 20.
When an aqueous solution of sodium hydroxide NaOH is added to an aqueous solution of chromiumIII nitrate CrNO33 a precipitate of chromiumIII. Notice the formula for the salt is. Lead II nitride 37 CoCO 3 cobalt II carbonate 38 CdSO 3 cadmium sulfite 39 CuNO 2 2 copper II nitrite 40 FeHCO 3 2 iron II bicarbonate Name the following chemical compounds.
Combined gas law formula. CopperII arsenate Cu 3 AsO 4 2 7610 36. Copper Cu Identify the oxidation state of the central metal ion shown in parantheses.
Cobalt is a naturally occurring element found in rocks soil water plants and animals. Required Cookies Technologies.
Cobalt Ii Carbonate Ccoo3 Chemspider
Search for Safety Data Sheet SDS Please enter the Product Number to find an SDS.

Cobalt ii old name. How to Enter Product Number. Business law firm with new name and expanded partner base. Cheatbook your source for Cheats Video game Cheat Codes and Game Hints Walkthroughs FAQ Games Trainer Games Guides Secrets cheatsbook.
This first video explains how the deal was discovered by NYT reporters. ALL Cane of the Tranquil. Transcripts of episodes of Critical Role.
5 mm 3 mm pkg of 3 ea. Devils Doorway was completed first but held back from release because of the nervousness of MGMs studio brass over the subject matter. All of the cation cards are printed with a corresponding number of holes the holes are actually punched in the cards to the appropriate oxidation state a ferrous cation would have 2 holes while a ferric cation would have 3 holes.
In Out. Immediately following the ions name. Rockets are a Hardmode post-Plantera type of ammunition which are mainly sold by the Cyborg.
The CRTranscript team provided the captioning for the show through Cornered 2x53 when captioning was taken over by Critical Role itself using a professional transcription service. March 26 2021 4. The word violet as a color name derives from the Middle English and Old French violete in turn from the Latin viola names of the violet flower.
He starred in one of the first post-World War II pro-Indian movies of the American cinema Devils Doorway 1950 his first western although Delmer Daves Broken Arrow 1950 was released one month before. Glyphosate complexes with cobalt as a dimer Coglyphosate23 in fifteen different stereoisomeric. Their decoration is mostly relief showing domestic life the hunt courting couples or drinking scenes.
5 mm 46 mm pkg of 3 ea. Instant at Level 46 WT. Cobalt Tin Grey Co2SnO4 is an inorganic pigment that is the reaction product of calcining a mixture of different amounts of both cobalt II oxide and tin III oxide.
Ascentis Express F5 27 μm Guard Cartridge. Some of the technologies we use are necessary for critical functions like security and site integrity account authentication security and privacy preferences internal site usage and maintenance data and to make the site work correctly for browsing and transactions. July 9 2021 4.
This child presented with microcephaly. Using InTune to gain access to a Windows Machine. Never built Delta HOSS cryogenic upper stage.
27 μm particle size L ID. They are homogeneously ionically entwined forming a crystalline spinel type matrix. Delta 2 and 3 rockets with a variety of GEM SRBs 4 meter Delta Cryogenic Second Stage modeled by Blowfish.
The first recorded use as a color name in English was in 1370. The 28L HO engine became fuel injected reducing output to 130 hp. It refers to the color of any different single wavelength of light on the short.
Federico Michele Sorrentino is the new Avocoms Equity Partner. On some old steins the relief figures are. After a few minutes of uploading the script which was a Cobalt Strike beacon payload we successfully got a beacon back and moved from cloud to on-premise.
FGvW Appoints two New Local Partners in Information Technology and Labour Law. Thus Fe2 is an ironII ion and Pb4 is a leadIV ion. The names of some simple cations are listed in Table II.
AgCl ____ silver chloride. Area of effect and the ability to destroy tiles. In this class you will ONLY be required to knowgive the modern Stock name Exercise.
The G20 or Group of 20 is an intergovernmental forum of 20 key players in the global economy including 19 countries and the European Union as part of a. A remarkable recent case of a three-month old infant suffering from molybdenum deficiency links several aspects of glyphosate toxicity together although glyphosate exposure was not considered as a possible cause in this case Boles et al 1993. Cobalt compounds are also used to color glass ceramics and paints and used as a drier for porcelain enamel and paintsRadioactive cobalt is used for.
IronIII oxide or ferric oxide Note. Transcripts of the first campaign and the first 42 episodes of Campaign 2 are found at Critical Role Transcripts. Searchable transcripts that include timestamps and.
The following year after a report by Sky News showed that cobalt mined by children was still being used in the companys devices Apple suspended purchases of hand-mined cobalt but once the. Similarly anion cards contain. In addition to the X platform GM also created a new line of engines for the Citation.
How to enter Lot Number COA Search. Thor-Delta rocket Almost all variants can be made and including Castor SRMs All Able and Delta upper stages with engines. Most launchers use Rockets as ammunition.
Ca2 is just calcium ion not calciumII ion because calcium only has one kind of stable cation. From SMP to YPOG. Please enter the Product.
Please enter the Product Number and Lot Number. All rocket types have two significant defining traits. 1H Blunt Atk Delay.
Table II Element Cation Systematic Name Old Style Name Cobalt Co2 CobaltII ion Cobaltous ion. The two-door Citation II was discontinued though the X-11 remained. The last Citation rolled off the assembly line on June 21 1985.
Rockets I and II have a small area of effect closest to that of a. Florida Department of State. 27 μm particle size L ID.
IronII fluoride or ferrous fluoride In Fe2O3 there are 3 O 2-ions per formula unit total charge -6 so here the ion is Fe3 two of them must add up to 6. Old and new earthenware steins range in body color from pale ivory to dark brown. We would like to show you a description here but the site wont allow us.
November 12 2021 4. Each card has the name in both the old system and the stock system and the charge of the ion printed on the card. Name the following compounds.
Abusing InTune with PowerZure. Cane of the Tranquil. November 19 2021 4.
Cobalt is used to produce alloys used in the manufacture of aircraft engines magnets grinding and cutting tools artificial hip and knee joints. Hunter Biden helped a Chinese conglomerate buy an American-owned cobalt mine in a 38 billion deal The purchase helped China gain the worlds largest deposit of the precious metal used to make batteries for electric vehicles and much more. This can also be abused purely through PowerZure using the New-AzureIntuneScript and Restart-AzureVM functions.
27 μm particle size L ID. The constitution may additionally have Al2O3 Fe2O3 andor NiO as modifiers that are used to adjust color hue and other properties that may be. Law firms dont have a crystal ball.
Florida Department of State Division of Corporations. 5 mm 21 mm pkg of 3 ea. The dashboard underwent a redesign with a horizontally-mounted radio and a new steering wheel.
How to Enter Product Number. Violet is closely associated with purpleIn optics violet is a spectral color. Juno II IV rockets.
BAR DWF TRL OGR HFL GNM IKS Cane of Harmony. -ous ending refers. Predominant background colors are either dark green cobalt blue or brown with maroon pink or black highlights.
Yaulp II Combat Casting Time. The Rocket I variant is also dropped by Plantera when it drops the Grenade Launcher.