Propan-2-ol can be dehydrated to give propene by heating it with an excess of concentrated sulphuric acid at about 170C. The acids arent written into the equation because they serve as catalysts.

File Propan 2 Ol Alt Png Wikimedia Commons
The tertiary structure only Question 5 A single 10 g piece of iron metal is completely submerged in 50 mL of 02 M nitric acid HNO 3 contained in a 100 mL beaker.
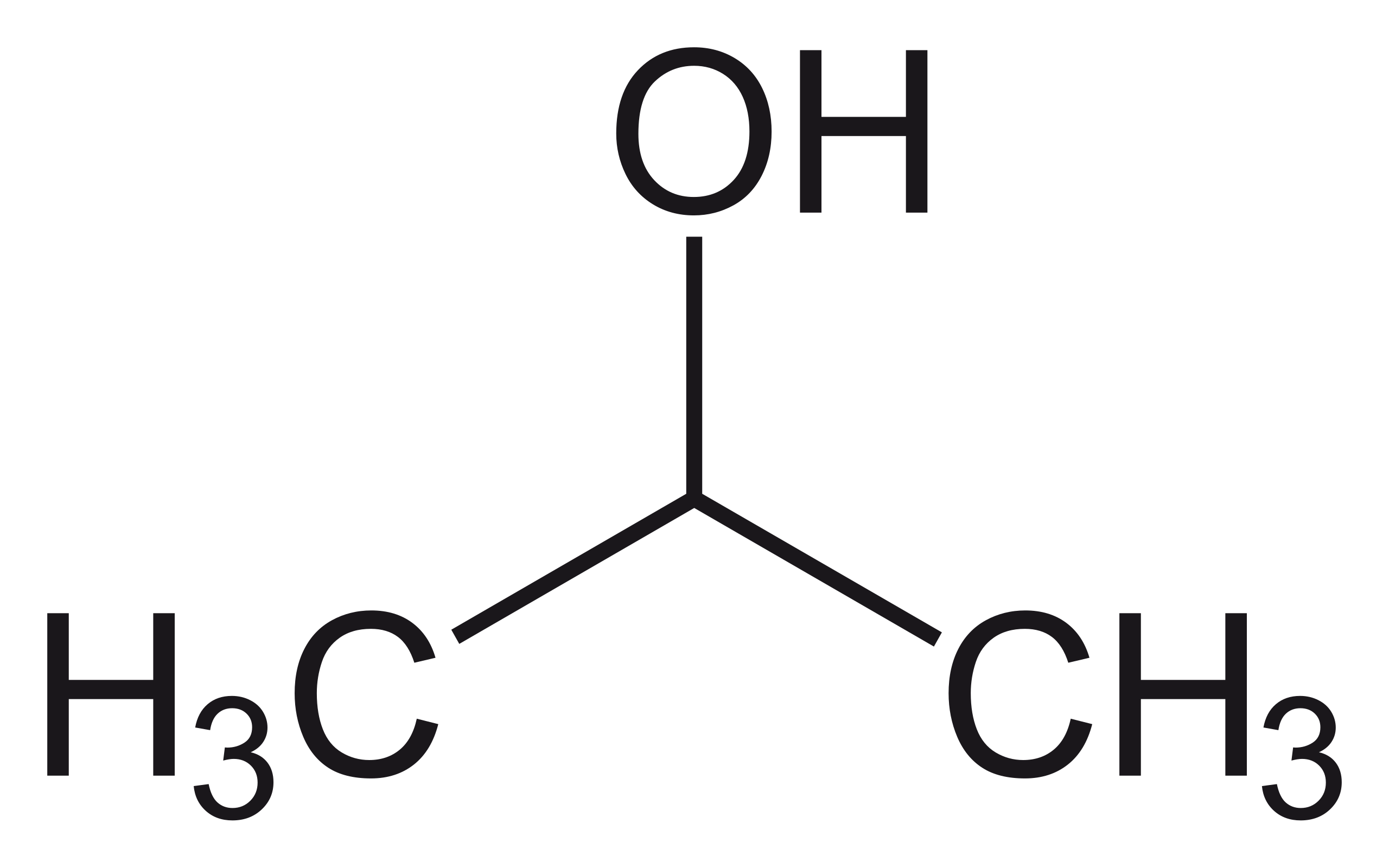
Structure of propan-2-ol. I CH 3 CH 2 CH 2 CH 2 OH ii 2-butanol iii 2-methyl-l-propanol Delhi 2012 Answer. The two propanol isomers consist of propan-1-ol and propan-2-ol also known as isopropyl alcohol which are distinguished by the placement of an oxygen atom either on the terminal carbon atom or the central carbon atom respectively. Active site structure Aeropyrum pernix.
Miscible with water alcohol ether chloroform Alfa Aesar 36644. So the condensed structural formula of propan-2-ol is CH_3CHOH. It has a role as a plant metabolite.
Elimination reactions in halogenoalkanes and alcohols when a displayed formula is required when a skeletal structure is. Table 1 Concentration of. FA9 can be propan-1-ol or propan-2-ol depending on whether the iodoform test test3 gives the yellow ppt of CHI3.
Disinfectant arbitrary units. The experiment is performed at standard laboratory conditions SLC. The dehydration of propan-2-ol.
DT-A61 A65 A68 A22 OUCMDZ-3118 and bingchenggensis ULS14 were reported in a roll during 2018 and 2019 Figure 2. Does this mean adding I2 in excess. A Illustrate the following name reactions.
When mixed with a more polar solvent such as 2-propanol propan-2-ol the mixture is sufficiently polar to carry the ibuprofen through the inner layer of the skin but not so polar that it will not dissolve ibuprofen. The use of propan-2-ol isopropanol isopropyl alcohol should be allowed for manufacturing the additives curcumin E 100 and paprika extract E 160 c in line with JECFA specifications as this particular use has been considered safe by the Authority 10. 1374-138 Food and Agriculture Organization of the United Nations Propan-2-ol.
Interpret evidence about chemical equilibrium systems oxidation and reduction properties and structure of organic materials and chemical synthesis and design to draw conclusions based on analysis. A total of 18 new indolocarbazole alkaloids 11 28 isolated from Streptomyces sp. Assume that an excess of oxidising agent is used.
Table 1 shows the students results. What is the IUPAC name for isopropyl alcohol. For propan-2-ol CH 3 CHOHCH 3 In most cases the use of sticks to represent C H bonds in a structure should not be penalised.
This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. A Lewis Structure electron dot diagram shows us how the bonding and non-bonding valence electrons are arranged around the atoms. What might FA10 and FA11 be.
This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. The primary structure only D. Draw the structure and name the product formed if the following alcohols are oxidized.
Take the test now. And structure of organic materials and chemical synthesis and design to identify trends patterns relationships limitations or uncertainty 4. In some molecules IUPAC names consider the positions where functional groups are located in the molecule.
1 - Phenyl - 2 - propanol. At 254nm 013 AU max. If you like you could write for example conc H 2 SO 4 over the top of the arrow.
IUPAC name Propan-2-ol. The three disinfectants used by the student were Lysol propan-2-ol and ammonia. Ibuprofen is synthesized from 2-methylpropylbenzene which can be made from compounds.
The use of ethanol in replacement of propan-2-ol in the manufacturing of gellan gum E 418 should be permitted where the final product. As an isopropyl group linked to a hydroxyl group it is the simplest example of a secondary alcohol where the alcohol carbon atom is attached to two other carbon atoms. The reaction that occurs is Fes 2HNO 3aq FeNO 3 2aq H 2g The rate of the reaction would most likely increase.
Some chemical compounds do not have common namesSome. A condensed structure uses parentheses to show that polyatomic groups within a formula are attached to the nearest non-hydrogen atom on the left. Identify any branches or side-chains in the condensed structural formula or.
The IUPAC name is directly related to its chemical structure. The exceptions to this when sticks will be penalised include structures in mechanisms where the C H bond is essential eg. In other words IUPAC names consider the functional groups side chains and other special bonding patterns in the molecule Examples.
The chemical structure C 3 H 8 O exists as several isomers of propanol as well as the isomer methoxyethane. Concentrated phosphoricV acid H 3 PO 4 can be used instead. Iii Propene to propan-2-ol.
4-06-00-03192 Beilstein Handbook. B Give a. It has a role as a plant metabolite.
Marking guide and solution. I Reimer-Tiemann Reaction ii Williamson Synthesis. It is a structural isomer of 1-propanol and.
Structureactivity relationship analysis of compounds 9 10 and seven known analogs showed C-6 chlorine as an essential pharmacophore in their cytotoxic activities. Propylene on esterification in the presence of sulphuric acid undergoes. Isopropyl alcohol IUPAC name propan-2-ol and also called isopropanol or 2-propanol is a colorless flammable chemical compound chemical formula CH 3 CHOHCH 3 with a strong odor.
Do you understand this. 2 The liquid culture the student transferred. 13776 Kaye Laby No longer updated Experimental Solubility.
At 230nm 025 AU max. Isopropyl Alcohol Structure C 3 H 8 O. Notice that the position of the -OH group is determined by where the HSO 4 group was attached.
Alpha-terpineol is a terpineol that is propan-2-ol substituted by a 4-methylcyclohex-3-en-1-yl group at position 2. A primary alcohol NAD an aldehyde NADH H catalytic mechanism involves a proton relay modulated by the coupled ionization of the active site Lys155Tyr151 pair and a NAD ribose 2-OH switch other active site residues are Ser138 and Trp144 ionization properties substrate binding overview Scaptodrosophila lebanonensis. But the condensed structure of ethanol is CH_3CH_2OH while for dimethyl ether the condensed structure is CH_3OCH_3.
Methoxyethane is also an isomer of C. Number of colonies of bacteria. Isopropyl Alcohol Synthesis- C 3 H 8 O.
You get propan-2-ol rather than propan-1-ol because of the way the sulphuric acid originally added across the double bond in propene. 0785 gmL Alfa Aesar L10181 36644 19397 22906 40983 39194 41840. Isopropyl Alcohol Structure C3H8O.
Draw the 2-dimensional full display structural formula for propan-2-ol 2-propanol which has the condensed structural formula or semi-structural formula CH 3 2 CHOH Step 1. Why is it so important to make sure you decant all of the isopropanol supernatant before. Molecular Weight Molar Mass.
CH 3 CHOHCH 3. More complicated alkyl hydrogensulphates react with water in exactly the same way. At 220nm 100 AU max.
Could someone please explain all reactions happening in the tests especially for FA11. For test 3 I am not sure why they want us to add I2 until the solution turns orangered. C 3 H 8 O.