M 1 V 1 M 2 V 2 16 molL 175 mL x 1000 mL x 028 M. I Calculate the magnitude of the heat absorbed by the solution during the dissolution process assuming that the specific heat capacity of the solution is 418 Jg.
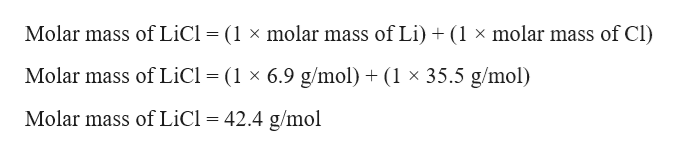
Answered Calculate The Molar Mass Of Each Bartleby
Here is a video which discusses how to calculate percent.

Molar mass of licl. Molar mass gmol Density Range of concentration. These NIR bands were assigned to the first overtone of surface hydroxyls and interlayer hydroxyls of LiOH respectively. The percent composition of the compound is.
Per unit weight there are more molecules of NH3 than of the gas mix that. NH 4 NO 3 s 2569. The volume of the solution is.
The unit asked for in the answer is mL acid. What is my theoretical yield of lithium chloride. 1295994 gmol anhydrous 23769 gmol hexahydrate Appearance yellow-brown crystals deliquescent anhydrous green crystals hexahydrate Odor.
NH 4 Brs 1678. The latter higher molecular ratio arises from the fact that the molar mass of NH3 is lower than the average for air ie. KC 2 H 3 O 2 s-1533.
This is an important distinction. NH 4 C 2 H 3 O 2 s-238. A Calculate the molar concentration of LiCl.
Do you know this. What is my percent yield. Molecular Weight Molar Mass.
Some other important properties of potassium dichromate are listed below. Anhydrous 675 g100 mL 25 C 876 g100 mL 100 C hexahydrate. The molar mass of glycine is required for this calculation and it is computed in the same fashion as its molecular mass.
The relevant given value is 730 g HCl. The molar mass of LiCl is 424 g mol 1. NaNO 3 s 2050.
If you dilute 175 mL of a 16 M solution of LiCl to 10 L determine the new concentration of the solution. 6829 g100 mL 0 C 7448 g100 mL 10 C 8425 g100 mL 25 C 887 g100 mL 40 C 12344 g100 mL 100 C Solubility. The provided mass of glycine 28 g is a bit more than one-third the molar mass 75 gmol so we would expect the computed result to be a bit.
Percent composition can be calculated the chemical formula of a compound or it can be determined experimentally. AgNO 3 s 2259. 878887 K Boiling point.
355 grams b I actually produced 6 grams of lithium chloride. Let the number moles of LiCl in 424 g mol 1 be n LiCl. The equilibrium of a saturated LiCl aqueous solution is shown below.
C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. The electrical conductivity of molten LiClKCleutSrCl2 mixtures has been measured from the liquidus temperature up to 11501220 K over the entire concentration range. The equilibrium of a saturated NaF.
What is the molar concentration of sodium ions in a 0750 M Na3PO4 solution. 605614 C 11211137 F. The molar mass of HCl 3646 gmol and the molarity of the solution 120 mol1000 mL provide the unit factors.
These NIR bands were assigned to. C 2 H 3 N. C 2 H 5 NO.
HBr HCl HClO4 KBr and NaCl are all classified as. 1274 K anhydrous 140 C hexahydrate Solubility in water. 355 gcm 3 anhydrous 192 gcm 3 hexahydrate Melting point.
Calculating the mass of Li and Cl Note. HCl HI H2SO4 LiCl and KI are all classified as. LiCl -s Li aq Cl aq At 20C the solubility of LiCl in water is 5500 gL.
Molar Mass of Frequently Calculated Chemicals. KNO 3 s 3489. C 3 H 6 O.
Acetic acid CH3CO2H formic acid HCO2H hydrofluoric acid HF ammonia NH3 and methylamine CH3NH2 are commonly classified as. For LiCl the student adds 1000 g of water initially at 150 C to a calorimeter and adds 100 g of LiCls stirring to dissolve. NaC 2 H 3 O 2 s-1732.
At a temperature of 0 o C the solubility of this compound in water corresponds to 49 grams per litre. The volume in the definition of molarity refers to the volume of the solution and not the volume of the solventThe reason for this is because one liter of solution usually contains either slightly more or slightly less than 1 liter of solvent. This compound is insoluble in alcohol and.
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. One mole of glycine C 2 H 5 O 2 N contains 2 moles of carbon 5 moles of hydrogen 2 moles of oxygen and 1 mole of nitrogen.
4239 gmol 1 Appearance white solid hygroscopic sharp Density. M Li m Li m LiCl since mass is always conserved. 1655 K Solubility in water.
K 2 Cr 2 O 7 has a crystalline reddish-orange appearance. H 2g18g x 100 111 O 16g18g x 100 889. Remember to keep the volume units consistent.
Accordingly 118 mol of LiCl will have 118 mol of Li and 118 mol of Cl. After the LiCl dissolves completely the maximum temperature reached by the solution is 356 C. LiOH KCl LiCl KOH a I began this reaction with 20 grams of lithium hydroxide.
B Calculate the molar concentration of Li and Cl-. 1382 C 2520 F. The density is estimated and the molar conductivity of these mixtures is calculated.
The molar mass of H_2O is 18 gmol The hydrogens make up 2g since each mole of hydrogen is 1g The oxygen makes up 16g. Soluble in hydrazine. For all molten mixtures the ln Λ vs 1T dependence has a nonlinear form.
Anhydrous LiOH showed two absorption bands at 7340 and 7171 cm1. Molar mass is used to describe the mass of one mole of a chemical compound. C Calculate the K sp for LiCl at 20C.
One mole of LiCl contains one mole of Li and one mole of Cl. C 2 H 4 O. Note that 1000 mL was used rather than 10 L.
LiOHH2O showed two absorption bands at 7137 and 6970 cm1. In chemistry molar concentration or molarity is defined as moles of solute per total liters of solution. We can summarize the two conversions as follows.
We select unit factors so as to cancel units. Both the specific and molar conductivities have negative. 1001 C 1834 F.
The hydration behavior of LiOH LiOHH2O and LiCl was observed by near-infrared NIR spectroscopy. 169 2 C 3 H 8 5 O 2 3 CO 2 4 H 2 O a If I start with 5 grams of C 3 H 8.