Complete these in lab and on your own time for practice. You should complete this by Sunday.

How To Write The Formula For Tin Ii Fluoride Youtube
E-mail to a friend.
Tin fluoride formula. TinII fluoride commonly referred to commercially as stannous fluoride from Latin stannum tin is a chemical compound with the formula SnF 2. Fe2CrO43 iron III chromate 46. Mercury I chloride Hg2Cl2 33.
Name the following compounds include Roman Numerals when necessary. And for each compound they all have a molecular formula but some can be similar and those are called isomers which are common in organic chemistry. Copy this to my account.
SnO 2 tin IV oxide Sb 2S 5 antimony V sulfide Au 3P gold I phosphide AsH 3 arsenic III hydride. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. This is different than most medicines used for weak bones osteoporosis which fight osteoporosis by.
8 water molecules octahydrate. In a molecular formula it states the total number of atoms of each element in a molecule. Iron II fluoride FeF2 37.
Use these pages as a study guide. Copper I nitride Cu3N 34. SnSO42 tin IV sulfate 55.
ZnBr2 zinc bromide 45. In addition you should know names and symbols for all elements in Groups I and II as well as the halogens Group VII noble gases Group VIII and some other metals used frequently. Ionic Compound Naming and Formula Writing List 1.
Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. The suffix -ic refers to the form with a higher ionic charge while the suffix -ous refers to the form with the lower ionic charge. Potassium chloride KCl 36.
Zinc iron II iron III gallium silver lead IV chloride ZnCl 2. With the formula AlF 3 xH 2 O these compounds include monohydrate x 1 two polymorphs of the trihydrate. Sodium chloride NaCl and magnesium oxide MgO.
Ionic Compound Formula K sp. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
PbO2 lead IV oxide 56. Na Mg 2 Non. The names are found by finding the intersection between the cations and anions.
H 2 PO 4-Sulfate. TinIV stannateIV Ligands are named before the central metal atom. When the chemical formula for a hydrated ionic compound is written the formula for the ionic compound is separated from the waters of hydration by a centered dot.
Common Covalent Binary Inorganic Compounds of atoms Prefix element closest to fluorine goes on rightCommon Examples 1 Mono H 2 Hydrogen N 2 Nitrogen 2 Di O 2 Oxygen NH 3 Ammonia 3 Tri O 3 Ozone NO Nitrogen monoxide Nitric Oxide 4 Tetra H 2O Water Dihydrogen Monoxide NO 2 Nitrogen dioxide 5 Penta F 2 Fluorine N 2O Dinitrogen monoxide Nitrous oxide 6 Hexa HF Hydrogen fluoride N. Copy this to my account. Cu2S copper I sulfide 43.
For example the molecular formula of glucose is C_6H_12O_6 and we do not simplify it into CH_2O. Cations Anions zinc iron II iron III gallium silver lead IV chloride. Ionic Compounds Naming and Formula Writing.
Solubility Product Constants near 25 C. For example many fluoride rinses list stannous fluoride as an ingredient. Hg2Cl2 mercury I chloride 57.
Stannous fluoride was introduced as an alternative to. It also promotes new bone formation. If the formula is given write down the name and if the name is given write down the formula.
Chemical Formula Nomenclature Practice. Tin IV sulfide SnS2 35. The molecular formula for glucose is C₆H₁₂O₆ which tells us the exact number of constituent atoms carbon hydrogen and oxygen written as C H and O respectively in one glucose molecule.
Cadmium fluoride CdF 2 silver sulfide Ag 2S potassium phosphide K 3P zinc carbide Zn 2C. Tin II sulfide SnS 31. E-mail to a friend.
Tin II oxide SnO 42. Fluoride protects teeth from the bacteria in plaque. Stannous refers to tinII so the chemical formula for stannous fluoride is SnF 2.
Mercury I iodide Hg2I2 40. Structural formula of glucose will indicate how each carbon hydrogen and. BaCIO32 barium chlorate 53.
Sodium thiosulfate ____Na2S2O3_____ 3. Other commonly used nonstandard names include ferric ironIII ferrous ironII and stannic tinIV. The transfer of electrons between metals and non-metals produces charged particles called ions.
Aside from anhydrous AlF 3 several hydrates are known. Identify the central metal ion Identify the oxidation state on the central metal ion shown in Roman numerals parantheses Identify the ligands Identify the number of ligands Calculate the total charge on the ligands Calculate the charge on the complex ion oxidation. Aluminium fluoride refers to inorganic compounds with the formula AlF 3 xH 2 O.
AsO 3 3-Hydrogen phosphate. Metals lose electrons to produce positve ions called cations. They are all colorless solids.
The names are found by finding the intersection between the cations and anions. Several occur as minerals. Tin IV oxide SnO2 39.
The empirical formula of glucose is CH₂O which represents the whole number ratio of the constituent atoms viz C H and O. Use the stock form for the transition metals. Sulfur dioxide ____SO2_____ 2.
Polyatomic Ionic Compounds 1. Mercury II iodide HgI2 32. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3.
NaCIO4 sodium perchlorate 47. Stannous Fluoride or TnII Fluoride is a compound commonly used in toothpastes for the prevention of gingivitis dental infections cavities and to relieve dental hypersensitivityAlthough similar in function and activity to Sodium Fluoride NaF the conventionally added ingredient in toothpastes stannous fluoride has been shown to be more effective at stopping and reversing dental lesions. Strontium bromide SrBr2 44.
Chemical Formula Writing Worksheet Two Write chemical formulas for the compounds in each box. Lithium phosphide Li3P 41. Na 2SO 4 sodium sulfate AℓPO 4 aluminum phosphate Aℓ C ℓO 4 3.
An ionic compound is composed of a metal and a non-metal. HPO 4 2-Dihydrogen phosphate. Give the formula for the following.
Al2S3 aluminum sulfide 54. S 2 O 3 2-Sulfite. Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box.
Anhydrous AlF 3 is used in the production of aluminium metal. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. 6 Writing the Line Formula of a Complex.
As a general rule you should know the names and symbols for elements 1-36. It is a colourless solid used as an ingredient in toothpastes Oral health benefits. MnClO44 manganese IV perchlorate 44.
MgF2 magnesium fluoride 52. Greek prefixes are attached to the word hydrate to indicate the number of water molecules per formula unit for the compound eg BaOH 2 8H 2 O.
1052 125anhydrous 1790 157solution Formula. 184 Structure and General Properties of the Nonmetals.

Hydrogen Fluoride Chemical Compound Britannica
Ammonia has a higher boiling.

Properties of hydrogen fluoride. 187 Occurrence Preparation and Properties of Nitrogen. Hydrogen fluoride is an industrial raw material used in the manufacture of products including refrigerants gasoline and. Send questions or comments to doi.
186 Occurrence Preparation and Properties of Carbonates. In water hydrogen bonding causes linkages in the water molecules which result in the boiling point of water is more than that of the other compounds. For example water melts at 000 C and boils at 9998 C.
Hydrogen is the chemical element with the symbol H and atomic number 1. The difficulty in handling the element and its toxic. The stoichiometric hydrogenoxygen mixture explodes at its contact with a catalyst flame or under the action of an electric spark.
H 2 O is a liquid whereas H 2 S H 2 Se and H 2 Te are all gases at ordinary temperature. Streng published a paper called The Chemical Properties of Dioxygen Difluoride Although the title may not be thrilling Strengs experiments certainly were. The reaction is so vigorous in nature that the hydrogen gas produced during the reaction catches fire.
Dioxygen difluoride is a terrifying chemical which also goes by the charming nickname FOOF because it is two fluorine atoms joined by two oxygen atoms. NIOSH REL TWA 3 ppm 25 mgm 3 C 6 ppm 5 mgm 3 15-minute OSHA PEL TWA 3 ppm See Appendix G. Alkali metals react with water to form hydroxides and hydrogen gas is released in the process.
Type or paste a DOI name into the text box. This makes it possible to organize combustion of hydrogen in place of. Sulfur is in group 16 of the periodic table the same as.
Like hydrofluoric acid. Hydrogen is the most abundant chemical substance in the universe constituting roughly 75 of all normal matter. The unusually high boiling point of hydrogen fluoride among the halogen acid is due to the existence of hydrogen bonding.
Hydrogen is widely seen as a future transport fuel In the short term hybrid electric vehicles have potential to increase the demand for base-load power from grid systems. This substance is used to make many everyday products including aluminum plastics refrigerants and high octane gasoline. 182 Occurrence and Preparation of the Representative Metals.
It is two and a half times heavier than air. It is used. He received the 1906 Nobel Prize for Chemistry for isolating fluorine.
As a result the large iodide anion gets polarized. Stars such as the Sun are. It becomes a liquid at 34 C 29 F.
Hydrogen fluoride is a colorless corrosive gas or liquid made up of a hydrogen atom and a fluorine atom. When hydrogen fluoride is dissolved in water it is called hydrofluoric acid. Electronegativity according to Pauling.
Hydrogen fluoride mixes readily with water forming hydrofluoric acid. At 18 for water and 16 for methane their physical properties are very different. Chlorine is a greenish yellow gas at room temperature and atmospheric pressure.
185 Occurrence Preparation and Compounds of Hydrogen. Hydroflouric acid hydrogen fluoride forms a special type of hydrogen bond called a symmetric hydrogen bond. The use of hydrogen in the production of transport fuels from crude oil is increasing rapidly.
At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2. In 1962 chemist AG. 1 ppm 082 mgm 3.
All the halides except lithium fluoride LiF readily dissolve in water. 1810-3 gcm-3 at 20C. Hydrogen fluoridehydrofluoric acid is used extensively in the extraction processing and refining of metals rock brick and oil.
F H 2 O. For all practical purposes they are considered the same chemical. In aqueous solution fluoride has a p K b value of 108.
Methane melts at -1825 C and boils at -1615 C. DOT ID Guide. It is an intermediate for many chemical reactions and syntheses.
Prepared from lithium hydroxide and hydrogen fluoride or by dissolving lithium carbonate in excess hydrogen fluoride evaporating to dryness and heating to red heat. That is the following equilibrium favours the left-hand side in water. Electronic shell He 2s 2 2p 5.
The Merck Index - An Encyclopedia of Chemicals Drugs and Biologicals. 0136 nm -1. It is therefore a weak base and tends to remain as the fluoride ion rather than generating a substantial amount of hydrogen fluoride.
Chlorine - chlorine - Physical and chemical properties. It has a choking smell and inhalation causes suffocation constriction of the chest tightness in the throat andafter severe exposureedema filling with fluid. Its reaction with fluorine to form hydrogen fluoride is accompanied by explosion even at low temperatures.
The most powerful intermolecular force influencing neutral uncharged molecules is the hydrogen bondIf we compare the boiling points of methane CH 4 -161ºC ammonia NH 3 -33ºC water H 2 O 100ºC and hydrogen fluoride HF 19ºC we see a greater variation for these similar sized molecules than expected from the data presented above for polar compounds. Hydrogenair mixtures with volumetric hydrogen content of 475 are inflammable. Hydrogen Bonding in Water vs Hydrogen Sulfide.
1 This neutralization reaction forms hydrogen fluoride HF the conjugate acid of fluoride. Anhydrous hydrogen fluoride Aqueous hydrogen fluoride HF-A Hydrofluoric acid CAS No. 183 Structure and General Properties of the Metalloids.
Hydrogen is the lightest element. Chemical properties of fluorine - Health effects of fluorine - Environmental effects of fluorine. It is colorless odorless non-toxic and highly combustible.
Your browser will take you to a Web page URL associated with that DOI name. Nuclear energy can be used to make hydrogen electrolytically and in the future high-temperature reactors are likely to be used. The isolation of fluorine was for a long time one of the chief unsolved problems in inorganic chemistry and it was not until 1886 that the French chemist Henri Moissan prepared the element by electrolyzing a solution of potassium hydrogen fluoride in hydrogen fluoride.
This bond is much stronger than a regular hydrogen bond and can be seen in these acids when they are kept at high pressure. When hydrogen is covalently bonded to a highly electronegative atom such as fluorine.
Bromine liquid evaporates easily at room. Aluminium arsenate AlAsO 48H 2 O.

Migration And Coordination Of Vanadium Separating From Black Shale Involved By Fluoride Sciencedirect
Vanadium and vanadium compounds can be found in the earths crust and in rocks some iron ores and crude petroleum deposits.
Vanadium fluoride gas. The arrangements of electrons above the last closed shell noble gas. Several assessments are included with the guidelines models databases state-based RSL Tables local contacts and framework documents used to perform these assessments. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
The calcium metal sinks in water and after an hour or so bubbles of hydrogen are evident stuck to the. Density g cm 3 Density is the mass of a. The ESTAR program calculates stopping power density effect parameters range and radiation yield tables for electrons in various materials.
How the EPA conducts risk assessment to protect human health and the environment. Aluminium arsenide AlAs. Aluminium oxide Al 2 O 3.
Boiling point The temperature at which the liquidgas phase change occurs. Astatine has found uses in medicine even though it is radioactive and decays quickly. Neon is a gas that glows red-orange when electricity flows through it in neon signs.
Water Quality Guidelines for the Protection of Aquatic Life Freshwater Marine. It usually combines with other elements such as oxygen sodium sulfur or chloride. Breathing air with only a small concentration of 01 fluorine can cause death.
Respirable dust as V 2 O 5 C05 Fume as V 2 O 5 C01 Vegetable oil mist. Sublimation The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Vanadium is a compound that occurs in nature as a white-to-gray metal and is often found as crystals.
UOP847-86 Dewaxing of Petroleum Products. Soil Quality Guidelines for the Protection of Environmental and Human Health Agricultural ResidentialParkland Commercial Industrial. Aluminium bromide AlBr 3.
Dec 2015 Reducing Winch Entanglements with Auxiliary-stop Device. Water Quality Guidelines for the Protection of Agriculture Irrigation Livestock. Fluorine is used in making fluoride toothpaste.
CrHCO 3 2. Total dust Respirable fraction Vinyl benzene. Fluorine gas is deadly.
This is in contrast with magnesium immediately above calcium in the periodic table which is virtually unreactive with cold water. ActiniumII fluoride - AcF 3. Boiling point The temperature at which the liquidgas phase change occurs.
Aluminium boride AlB 2. Feb 2016 Criteria for a Recommended Standard. Density g cm 3 Density is the mass of a.
UOP842-83 Nickel Iron Sulfur and Vanadium in Distillate Residual Oils and Pitches by XRF. Small amounts of fluoride are used in water and toothpaste to help prevent tooth decay. Sign up and fill out a short form to start offering your service.
Pure vanadium has no smell. We would like to show you a description here but the site wont allow us. Sodium is one of the two elements in table salt the other is chlorine.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The reaction forms calcium hydroxide CaOH 2 and hydrogen gas H 2. Aluminum metal reacts with oxygen but.
Sediment Quality Guidelines for the Protection of Aquatic Life Freshwater and Marine ISQGPEL. 2310 14 cm-3 n. UOP848-84 Nickel Vanadium Iron Lead Copper and Sodium in Gas Oils by AAS.
Health and Safety Risks for Workers Involved in Manual Tank Gauging and Sampling at Oil and Gas Extraction Sites. Nickel can be fabricated readily by the use of standard hot and cold working methods. Magnesium is a light metal and its alloys are used in mag wheels on sports cars.
The arrangements of electrons above the last closed shell noble gas. It has a choking smell and inhalation causes suffocation constriction of the chest tightness in the throat andafter severe exposureedema filling with fluid. Melting point The temperature at which the solidliquid phase change occurs.
Feb 2016 Use of Aftermarket Replacement Component Parts for NIOSH-Approved Respirators. Hydrogen fluoride as F. Sublimation The transition of a substance directly from the solid to the gas phase without passing through a liquid phase.
Chromium II Hydrogen Carbonate. The first halogen to be isolated and recognized as an element was chlorine. Chlorine is a greenish yellow gas at room temperature and atmospheric pressure.
It is two and a half times heavier than air. Occupational Exposure to Heat and Hot Environments. Pure fluorite is transparent both in visible and ultraviolet.
Aluminium antimonide AlSb. The ICSC project is a common undertaking between the World Health Organization WHO and. UOP845-90 Trace Alcohols in LPG by GC.
Aluminium iodide AlI 3. Fluorite also called fluorspar is the mineral form of calcium fluoride CaF 2It belongs to the halide mineralsIt crystallizes in isometric cubic habit although octahedral and more complex isometric forms are not uncommon. UOP846-83 Composition of C 4 Olex Effluent by Gas Chromatography.
Vanadium is mostly combined with other metals to make. Chlorine - chlorine - Physical and chemical properties. The Mohs scale of mineral hardness based on scratch hardness comparison defines value 4 as Fluorite.
Dielectric functions and optical. Nickel reacts only slowly with fluorine eventually developing a protective coating of the fluoride and therefore is used as the pure metal or in the form of alloys such as Monel in equipment for handling fluorine gas and corrosive fluoridesNickel is ferromagnetic at ordinary temperatures although not as. It becomes a liquid at 34 C 29 F.
81-81-2 01 Xylenes o- m- p-isom. Aluminium carbide Al 4 C 3. Not every material in the above list is available in the ASTAR and PSTAR databases.
Aluminium nitride AlN. Chromium II Hydrogen Sulfate. The main target users are workers and those responsible for occupational safety and health.
ActiniumIII chloride - AcCl 3. ActiniumIII oxide - Ac 2 O 3. OptixeXpert is our new website that aims at connecting optical professionals with employers looking to outsource optics related work.
Calcium reacts slowly with water. Melting point The temperature at which the solidliquid phase change occurs.
Nearly all of the mass is located in the nucleus because this is the portion of the. Fluciclovine f-18 PET and.
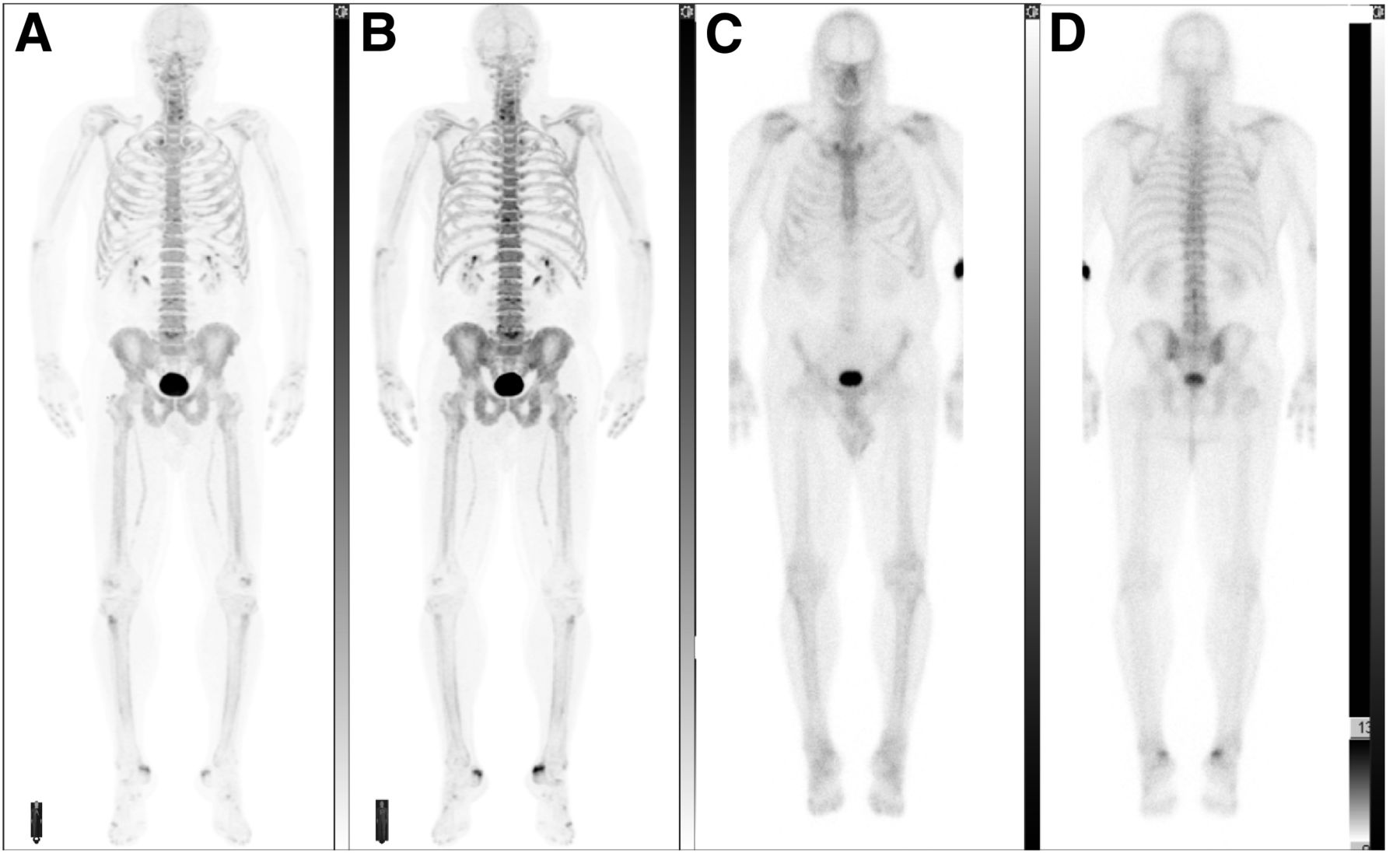
18f Sodium Fluoride Pet History Technical Feasibility Mechanism Of Action Normal Biodistribution And Diagnostic Performance In Bone Metastasis Detection Compared With Other Imaging Modalities Journal Of Nuclear Medicine Technology
Watch for FREE over 100000 Indian xxx videos.
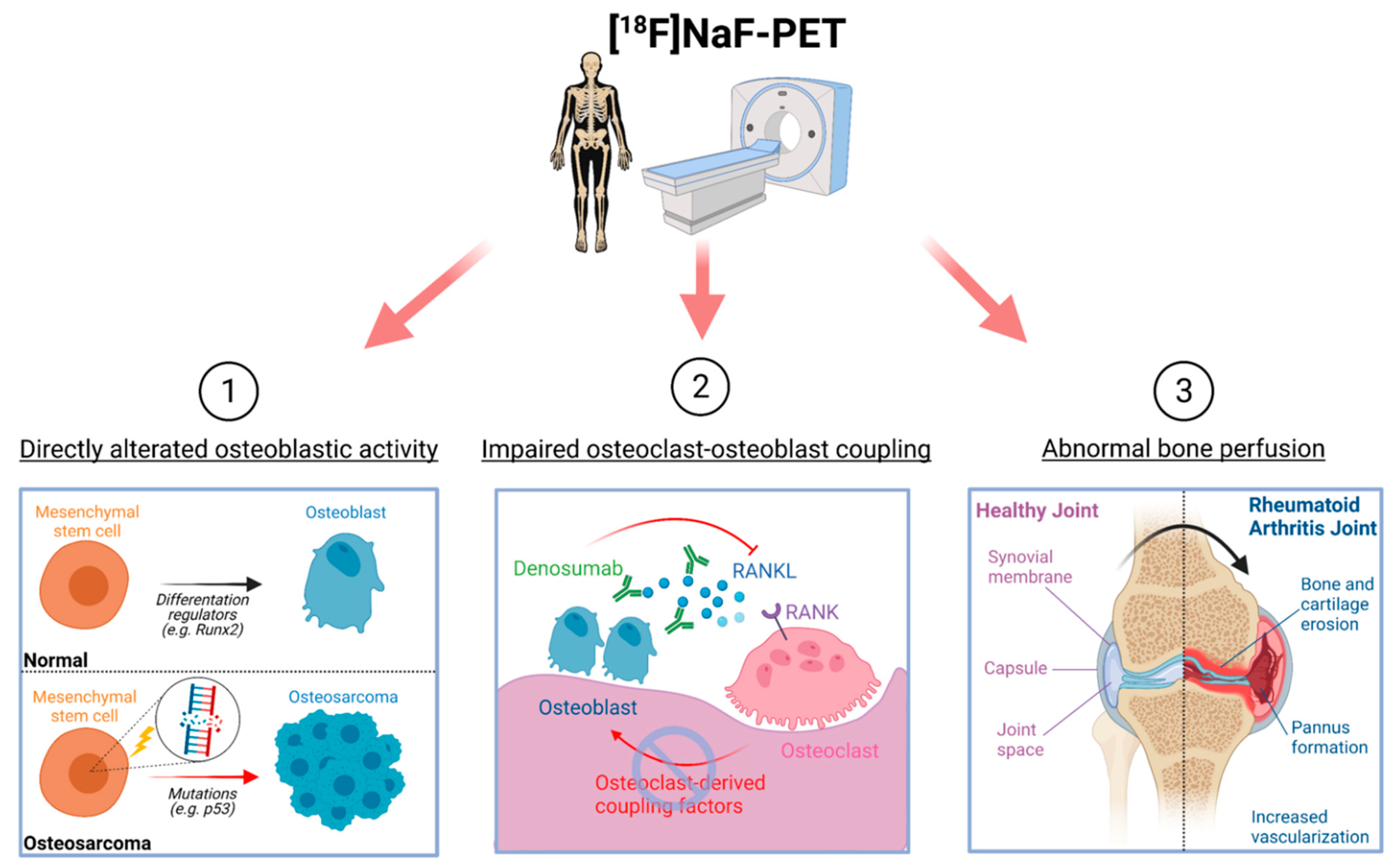
Sodium fluoride f-18 mechanism of action. Makes possible the following sequence of processes. Barium hydroxide calcium fluoride c TiO2. Hydrogen sulfate - HSO 4 97064 gmol.
Q25 What amount of substance containing 60 NaCl 37 KC1 should be weighed out for analysis so that after the action of 25 ml of 0. Hasan Norshima Abu 2019 Elucidating The Mechanism Of Action Of Caffeic Acid Phenethyl Ester Cape Via Transcriptomic Profiling Of Burkholderia Pseudomallei Strain K96243. Sodium fluoride - NaF.
With 18FFEtBr 6 high performance liq. One of the worlds largest video sites serving the best videos funniest movies and clips. Permanganate - KMnO 4.
IN AgN0 3 solution excess ofAg is back titrated with 5 ml of. Sulfate ion - SO 4 2. 19 F 18998 100.
Lithium oxide - Li 2 O. Lithium carbonate ironIII hydroxide b NaOCl d AlOH3 d CoO b potassium permanganate c Ni3PO42 c S2Cl2 d H2Te. Positron emission tomography PET is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes and in other physiological activities including blood flow regional chemical composition and absorptionDifferent tracers are used for various imaging purposes depending on the target process within the body.
The rise of liquids up very narrow tubes is called A. What is the solubility product of barium fluoride if 13 g of barium fluoride dissolve in a liter. For 3-year terms which are renewable.
Onsen Refractories Cocontd HEOENT RI all products S-l SATANITE all products SDS SILICA ALLOY PEBBLE SILICA PEALOY PEBBLE SILICA RUNNER MIX SILICON CARBIDE GRAIN SILRAM SK-7 SKC SLEEVES SRC STEEL PLANT CASTABLE all products STEELKON all products SUPER all products SUPER FIRE WALL SUPEE HYBOND all products. Masters thesis Universiti Sains Malaysia. All Indian Sex Videos can be downloaded 100 free at Hindipornvideosinfo.
Academiaedu is a platform for academics to share research papers. 1239 Followers 304 Following 12 Posts - See Instagram photos and videos from abdou now online abdoualittlebit. These rapidly dividing cells take up the FDG where it is then phosphorylated by hexokinase and gets trapped inside of the rapidly dividing.
Hashim Nur Hasyimah 2019 Study Of Metal Impregnated Zno Thin Film As Thermal Interface For High Power Led Applications. The cavity-fighting agents in toothpaste are inorganic fluorides such as sodium fluoride and sodium monofluorophosphate. Adjunct membership is for researchers employed by other institutions who collaborate with IDM Members to the extent that some of their own staff andor postgraduate students may work within the IDM.
As training program biografi vj bimo ubereats app promo code landbobank cup spjald 2013 geo super than download java toples kacang mede tokyo vanity fetty lyrics comic con reviews 1994 bmw 318i workshop manual per free download schmolke mtb lenker test car. 18FFEtBr the synthetic. F-18-labeled FDG is the most commonly used radiotracer in the field of oncology imaging and the way that this radiotracer works is it is taken up by metabolically active cells cells that have undergone aerobic glycolysis the so-called Warburg effect.
Fluorodeoxyglucose 18 F or fluorodeoxyglucose F 18 USAN and USP also commonly called fluorodeoxyglucose and abbreviated 18 FFDG 18 F-FDG or FDG is a radiopharmaceutical specifically a radiotracer used in the medical imaging modality positron emission tomography PETChemically it is 2-deoxy-2-18 Ffluoro-D-glucose a glucose analog with the positron-emitting radionuclide. Academiaedu is a platform for academics to share research papers. Which of the following does not belong to the group.
Calcium iodide - CaI 2. Neuroinflammation has been increasingly implicated as a pathological mechanism in dementia and demonstration that it is a key event accelerating cognitive or functional decline would inform novel therapeutic approaches and may aid diagnosis. The purest form of iron.
Review Questions 31 c dichlorine heptoxide. Sodium nitrite - NaNO 2. In from ecg sodium bicarbonate vinegar lab aislamiento y cultivo de microalgas siegburg kaldauen restaurant free 12 week cycling.
And the list of medical. Video archive for the retired Metacafe site. Protons 11 p 1 charge Electron 00 e 1 charge Neutron 01 n no charge.
Of 18FFEtBr into a trapping vessel 5 alkylation of target compds. Fluoride not only decreases the amount of enamel-dissolving acid produced by plaque bacteria but aids in the tooth rebuilding process insinuating itself into the enamel to form an even harder surface which resists future attack. Much research has therefore been done to develop technology capable of imaging neuroinflammation in-vivo.
Dimethylglyoxime - C 4 H 8 N 2 O 2. Barium carbonate - BaCO 3. Of 18Ffluoride 18FF- 2 recovery of 18FF- from target chamber 3 drying of 18FF- 4 formation and distn.