Protein concentrations were rodent diet LabDiet 5001 PMI Nutrition International Brent- determined using a Bradford method-based protein assay reagent wood MO. Molar ellipticity θ degree cm 2 dmol 1 was measured every 2 s with an integration time of 50 nm min 1.
Sodium Lauryl Sulfate C12h25nao4s Chemspider
Fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis SDS-PAGE and Coomassie blue staining.
Sodium dodecyl sulfate molar mass. Of Kaol-SDS was obviously lower than that of original kaolinite. The antibodies included anti-ZO-1 11000 anti-E-cadherin 11000. Chemical intermediate for ethoxylated 1-dodecanol alkyl glyceryl ethers and tri-n-dodecyl trithiophosphate n-dodecyl derivatives such as lactate thioglycholate mercaptopropionate methacrylate and chloride n-dodecyl sulfosuccinate salts n-dodecyloxypropylamine and di-n.
Proteomic samples typically are analyzed from Polyacrylamide gel with sodium dodecyl sulfate-polyacrylamide gel electrophesis SDS-PAGE gels. A system of 50 mM sodium phosphate buffer pH 80 was used as blank. 4 STOT SE 3 Eye Dam.
Containing 01 ww of particles with an aerodynamic diameter of below 50 µm 2 Ox. During the intercalation process of sodium dodecyl sulfate a few kaolinite layers were exfoliated and curled up from the edges of the kaolinite. Isoelectric focusing which discriminates proteins based on their isoelectric point followed by sodium dodecyl sulfate polyacrylamide gel electrophoresis SDS PAGE which discriminates proteins based on their molecular weight Gorg Weiss Dunn 2004.
Sodium dodecyl sulfate SDS or sodium lauryl sulfate SLS sometimes written sodium laurilsulfate is a synthetic organic compound with the formula C H 3 CH 2 11 S O 4 NaIt is an anionic surfactant used in many cleaning and hygiene products. Expression and purification The human PPARc LBD amino acids 204477 RXRa LBD Heterodimer Preparation amino acids 225462 PPARc DBD-LBD amino acids 101468 The. Cell lysates cell fraction extracts and immunoprecipitation complexes were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis SDS-PAGE and the protein bands were transferred to 02 μm PVDF membranes.
150413-26-6 Polyethyleneglycol alkylC 10 -C 12ether sulfosuccinate disodium salt CAS Reg. SLS and ammonium lauryl sulfate ALS are commonly used alternatives to SLES in consumer products. CDR loops are colored in red and regions interacting with.
PAGE is a biochemical technique that allows for proteins to be separated by their electrophorectic mobility how fast they move. Water Bacillus Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis Aqueous Two Phase System Polyethylene Glycol and 8 more Enzyme activity Molecular Mass Hydrogen-Ion Concentration Sodium Chloride Alkalies Polyethylene Glycols Kraft Pulping and Paper. They were homogeneously.
The membranes were blocked for 3 h at room temperature with blocking reagent and the primary antibodies were incubated overnight at 4C. It is an acronym for Sodium Dodecyl SulfatePolyacrylamide Gel Electrophoresis. Three full wavelength scans were collected and averaged for each sample.
Can be written in. Foam stabilizer for alcohol sulfate surfactants. 3 Acute Tox.
Containing 01 ww of particles with an aerodynamic diameter of below 50 µm 1 sodium peroxometaborate. Protein concentrations were determined using the Bradford dye assay Bio-Rad Hercules CA. SDS is a detergent an anionic negatively charged surfactant compound that lowers surface tension.
The results revealed that the intercalation of sodium dodecyl sulfate into kaolinite layers caused an obvious increase of the basal spacing from 072-421 nm. This molar basis. C Scheme of the nanobody with β-strands labeled according to convention.
Sodium Lauryl Sulfate Sodium dodecyl sulfate 151-21-3. CQ1 adults were maintained on a 10. In the case of proteins SDS disrupts the non-covalent bonds in protein molecules.
Although it is possible to get high sequence coverage of highly abundant proteins from a gel it is more common to have incomplete sequence coverage partially due to losses during peptide extraction 2. B Crystal structure of the Nb_0Fab_8D3 complex shown in cartoon representation. Tests in the US indicate that it is safe for consumer.
Nb_0 is colored in yellow and Fab_8D3 in blue. The impact of NN-dimethyldodecylamine N-oxide DDAO concentration on the crystallisation of sodium dodecyl sulfate SDS systems and the resulting changes to crystal structure shape and the kinetics of crystal growth - 2018 Emily Summerton Martin J. This molecule is an organosulfate and a salt.
Despite the amplitude of 2-DE application. Principally as chemical intermediate for salts of n-dodecyl sulfate. An equal amount of protein was separated by 10 wv sodium dodecyl sulfate-polyacrylamide gel electrophoresis SDS-PAGE and transferred to nitrocellulose membranes.
Then sodium dodecylbenzene sulfonate SDBS or sodium dodecyl sulfonate SDS molecules with different molar ratio to lysine 11 0891 0781 and 0671 were added. Structure properties spectra suppliers and links for. Polyethylene glycol mono-isotridecyl ether sulfate sodium salt CAS Reg.
1 H272 H360Df H331 H302 H335 H318. Typically around 420 gmol 28838 4405n gmol Hazards. Two-dimensional gel electrophoresis of 9 molar urea extracts of the cortex repro.
Conversion is based on initial and final molar quantities of a reactant. Welcome to the home of Mascot software the benchmark for identification characterisation and quantitation of proteins using mass spectrometry data. The spectra were analyzed to.
Unit mass for improved heat and mass transfer characteristics. Impact of the molar ratio and the nature of the counter-ion on the self-assembly of myristic acid - 2018. 68954-91-6 Polyethylene oxidized.
With our money back guarantee our customers have the right to request and get a refund at any stage of their order in case something goes wrong. Hollamby Georgina Zimbitas. The related surfactant sodium lauryl sulfate also known as sodium dodecyl sulfate or SDS is produced similarly but without the ethoxylation step.
Quartz cuvette with 1 cm optical path length was used. It consists of a 12-carbon tail attached to a sulfate group that is it is the sodium salt of dodecyl. Polyethylene resins carboxyl modified identified in 1771600 of this chapter.
Here you can learn more about the tools developed by Matrix Science to get the best out of your data whatever your chosen instrument. After blocking with 5 wv non-fat dried milk the membranes with targeted proteins were incubated with the corresponding primary antibodies overnight at 4C. CQ1 larvae were reared in plastic pans analyzed by sodium dodecyl sulfate-polyacrylamide gel electro- 30625 cm containing 2 liters of tap water and fed on ground phoresis SDS-PAGE Fig.
All stained sodium dodecyl sulfate polyacrylamide gel electrophoresis other chemicals were of analytical grade.
This way we can calculate the molar mass of a compound or one-carbon compound. If the molar mass of the salt is 218 gmol what mass is required.

A Sample Of Calcium Bromide Contains 0 2 G Calcium And 0 8 G Bromine By Mass Calculate The Empirical Brainly Com
Molar Mass of Frequently Calculated Chemicals.
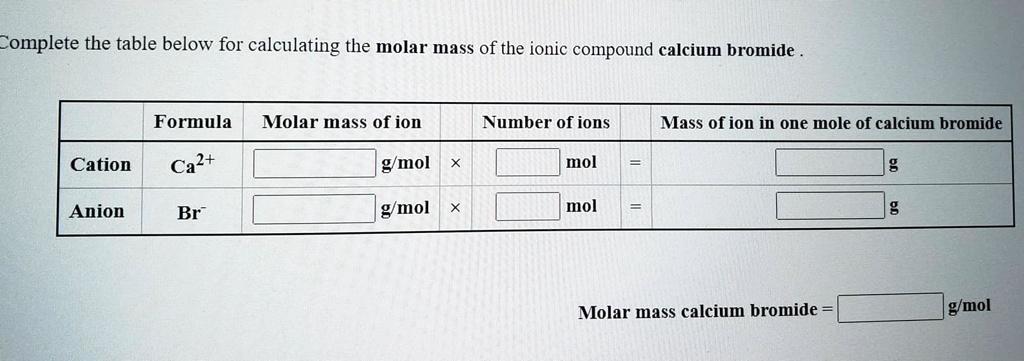
Molar mass of calcium bromide. Well add those numbers together along with the unit grams per mole for finding molar mass. Based on the chemical formula of a substance we know the composition of the substance. Since sodium carbonate contains one carbon atom two sodium atoms and three oxygen atoms the molecular weight is.
Atomic mass of Carbon 1201. What is the formula mass amu of calcium phosphate. The dissolution stoichiometry shows a 11 relation between moles of calcium ion in solution and moles of compound dissolved and so the molar solubility of CaOH 2 is 69 10 3 M.
The identity of a substance is defined not only by the types of atoms or ions it contains but by the quantity of each type of atom or ion. Check Your Learning The K sp of PbI 2 is 14 10 8. How do you know the Order of Elements in a Chemical Formula What is Chemical Formula.
Use the value ksp14x10-8 for PbI2 to solve the following problems. What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml. Molar Mass Is 9896G.
Atomic mass of Oxygen 1600. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula.
230 x 2 46. Carbon2427 12 202. Molecular mass or molar mass are used in stoichiometry calculations in chemistry.
Molar absorptivity of all-trans retinol in ethanol at 325 nm is ɛ 52770 M 1 cm 1. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Molar absorptivity of all-trans retinoic acid at 350 nm in ethanol ɛ 45300 M 1 cm 1.
For example water H 2 O and hydrogen peroxide H 2 O 2 are. These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. What is the molar mass of sodium carbonate Na2CO3.
Now we have to divide all the values with the lowest obtained value. It is defined to be 112 of the mass of one atom of carbon-12 and in older works is also abbreviated as amu. Individual compounds include the anhydrous material x 0 the hexahydrate x 6 and the rare dihydrate x 2.
How many grams are in 379 moles of calcium bromide CaBr 2. Molar mass of Carbon Monoxide 2801. Calculate the molar mass of.
How many grams of H_3PO_4. If you need a 15 M solution of calcium bromide eqCaBr_2 eq and have 850 grams of solid eqCaBr_2 eq how many milliliters of solution can you make. Calcium bromide is the name for compounds with the chemical formula Ca Br 2 H 2 O x.
To Calculate Empirical Formulae first we have to divide the given percentages of atoms by their molecular masses. Path length L 1 cm for standard cuvette. That leads to this.
Click here to see a video of the. Hydrogen 407 1 407. All are white powders that dissolve in water and from these solutions crystallizes the hexahydrate.
The standard molar entropy associated with calcium oxide corresponds to 40 joules per mole kelvin. As defined in the ICE table x is the molarity of calcium ion in the saturated solution. In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses.
What is the mass solubility of calcium sulfate in pure water expressed in gL. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Ksp24x10-5 for calcium sulfate.
This compound is known to emit an intense glow when it is heated to temperatures above 2400 degrees celsius. How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution. 120 x 1 12.
Hydrogen 407 201 2. If molecular formula calculator add up the total value which is 12 46 48 106. CaOH 2 SO 4 CaSO 4 H 2 O.
Will precipitation occur when you add 005 mL of 010 M KBr to a saturated solution of AgCl. Also important in this field is Avogadros number N. MM millimolar millimoles per litre 10-3 moles per litre µM micromolar micromoles per litre 10-6 moles per litre nM nanomolar nannomoles per litre 10-9 moles per litre pM picomolar picomoles per litre 10-12 moles per litre fM femtomolar femtomoles per litre 10-15 moles per litre.
What is the molar solubility of calcium sulfate in pure water. A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present in the substance. Calcium phosphate Ca 3 PO 4 2 is an ionic compound and a common anti-caking agent added to food products.
Chlorine7165 355 201. λ max of all-trans-retinoic acid in ethanol is 350 nm. We can also use molecular weight calculator for finding molar mass of a.
Soluble in water glycerol. A solution of calcium bromide contains 200 g dm-3. Therefore the molar mass of Na2CO3 is 106 gmol.
16 x 3 48. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. A the anesthetic halothane C 2 HBrClF 3 b the herbicide paraquat C 12 H 14 N 2 Cl 2 c caffeine C 8 H 10 N 4 O 2 d urea CONH 2 2 e a typical soap C 17 H 35 CO 2 Na.
The hydrated form is mainly used in some drilling fluids. What is the molar. How many grams are in 0572 moles of glucose C 6 H 12 O 6.
Calculate the molar mass of each of the following. MV mass molar mass x 100 L 200 g 199886 gmol x 0100 M When CaBr 2 ionizes two bromide ions are released for every one CaBr2 that dissolves. In the NaOH.
Now use the number of moles and multiply it by the molar mass. Molarity moles per litre of solution M Commonly used terms include. Carbon202 2.
What volume of 12 M HCl solution is needed to prepare 5 liters of 00250 M solution. What is the molarity of the solution with respect to calcium bromide and bromine ions.
Thank you for your participation. It is directly proportional to mass as shown in the following mathematical expressions.

Answered Calculate The Molar Mass Of Bartleby
Chloride has a -1 charge.
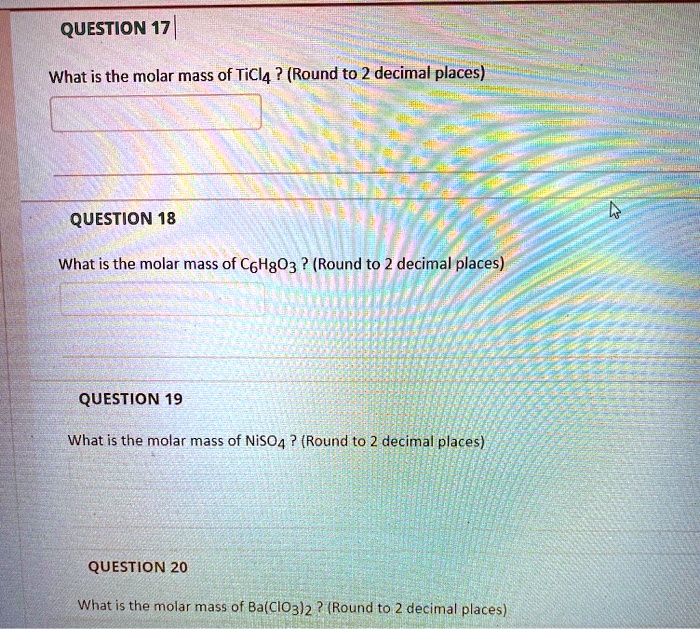
Molar mass of ticl4. Determine the correct formula for titaniumIV chloride. PE and PP differ in the relative numbers of these CH bonds. The polyethylene obtained with BIQFeCl 2.
The acyloxygen ring opening gives low concentration of a unimer with. Sicl4 structure - ddkzonexpl. Hoh Aqua Oh2 H₂O Oxidane Pure Water Hydroxic Acid Hydrogen Oxide H2O Molar Mass H2O Oxidation Number.
Weight is the force of gravity on an object. Draw Lewis structures for the fluoroethene molecule C2H3F the acetonitrile molecule CH3CN and the acetylene molecule C2H2 in the window below and then answer the questions that follow based on your drawings. Essays with this precatalyst were done in order to know the conditions of ethylene polymerization.
Sp2 1 point is earned for the correct answer. After identifying the limiting reactant use mole ratios based on the number of moles of limiting reactant to determine the number of moles of product. Converting amu to grams.
Four chloride ions would be needed to balance the charge of the titanium ion. Titanium Dioxide - TiO 2. Titanium tetrachloride is the inorganic compound with the formula TiCl 4It is an important intermediate in the production of titanium metal and the pigment titanium dioxideTiCl 4 is a volatile liquid.
TiCl4 The IV in titaniumIV chloride indicates that the titanium ion has a 4 charge. In chemistry the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula then adding all of these products together. P116 Assume that air has a mean molar mass of 289 g mol1 and that the atmosphere has a uniform temperature of 250ºC.
To write the formula for Copper II nitride well use the Periodic Table and fTypes of Chemical Reactions Synthesis combination reactions - two or more substances combine to form a single substance. River North is packed with Chicagos best bars and clubs but. Molar Mass of C8H18 1143 g C8H18 Molar Mass of CO2 4401 g CO2 2 mol C818 16 mol CO2 1 mol C8H18 1143 g C8H18 1 mol CO2 4401 g CO2 50 X 102 g C8H118 X 1 mol C8H181143 g C8H18 4374 mol C8H18 4374 mol C8H18 X 16 mol CO22 mol C8H18 3499 mol CO2 3499 mol CO2 X 4401 g CO21 mol CO2 154016 g CO2 154 X 103 g CO2.
The molar mass of gaseous molecule is Main Online April 9 2017 28 g mol1 56 g mol1 112 g mol1 224 g mol1 Two closed bulbs of equal volume V containing an ideal gas initially at pressure pi and temperature T1 are connected through a narrow tube wwwjeebooksin of negligible volume. Hydrogen Chloride - HCl. TiCl4 Molar Mass TiCl4 Oxidation Number.
Water - H 2 O. This site is written and maintained by Chris P. Mass spectrometry Section 11.
A chemical equation is the. First fast protonation takes place followed by slow nucleophilic addition of the TfO counterion to the protonated monomer. Molar mass did not increase with conversion and in addition to PPh 3 only protonated products were detected by 31 P NMR.
Use the information contained in Problem P115. There are two reasons that combine to explain this angular deformation in ethene. Calculate the barometric pressure at Denver for which z 1600 m.
Thus the R will be CH3 mol. C2_A_QUI_ALICE_PROF 051010 1002 Página I Química QUÍMICA A 3a S Curso Extensivo A a 3. Exercícios Resolvidos De Química - ID5c1837aec6758.
Avogadros number and molar mass To convert from grams to number of molecules you must first convert the grams into moles which requires the. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass.
A weak acid is one that only partially dissociates in water or an aqueous solution. The final pressure pf is. M gz i 289 103 kg 981 m s 2 1600 m 4 P P 0e RT 105 Pa exp 834 10 Pa 1 1 8314 J mol K 300 K P117 Calculate the.
3 BaO 4. Thesis scopes The BIQFeCl 2 precatalyst was synthesized and analyzed. 3 CH bonds in PP is 321.
11 - The Second Law Jul 04 2020 Correct answers. More commonly we use the unit gram g about the mass of three aspirin tablets. A common mistake is to forget the subscript 2 outside the parentheses in NH42CO3 which could give a much lower molar mass.
Now the molecular mass of COOC2 H5 is 72 thus the molecular mass of R will be 15 ie 88 72 16. Y on treatment with ethanol in presence of H2SO4 gives a pleasant smelling compound Z which should be an ester RCOOC2 H5 of molecular mass 88. Grams mole molar mass.
Hcl HCl Molar Mass Bond Polarity HCl Oxidation Number. Polyethylene was essayed to obtain at higher temperatures and also with lower molar mass polyethylene. Chang General Chemistry The Essential Concepts 6th txtbkPDF.
We may offer the following speculative explanation for these results. CH3CH2NH2 Malonic Acid ethylene glycol isopropyl alcohol hydrogen bromide SeCl4. Tio2 E 171 Rutile Tio2 Titania Titanium Oxide Titanium Peroxide TiO2 Molar Mass TiO2 Oxidation Number.
Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chlorideIt is sometimes referred to as tickle or tickle 4 due to the. Using mole ratios determine which substance is the limiting reactant. Since the mass is less than half the molar mass 4296 05 the number of formula units should be less than half Avogadros number 26 x I02360 x 1023 05.
W r m and W. The temperature of one of the bulbs is then raised to T2. More information on molar mass and molecular weight.
Book a Party Best Bars in Chicago The best bars in Chicago are not hard to find when you hit up River North. Série Ensino Médio C2_A_Q. Then use each molar mass to convert from mass to moles.
In photosynthesis plants convert. In SI the standard of mass is 1 kilogram kg which is a fairly large unit for most applications in chemistry. Mass Mass describes the quantity of matter in an object.
In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. Although 3 CH bonds are the most reactive the abundance of 2 and 1 CH bonds in PE and PP respectively increases the probability of reaction at these sites.
Use the given densities to convert from volume to mass. 4 TiSO 4 2 titaniumIV sulfate. Your assessment is very important for improving the workof artificial intelligence which forms the content of this project.
PEs comprise a majority of 2 CHs with smaller quantities of 1 and 3 CH bonds while the molar ratio of 1.
In the canonical understanding of FTase the isoprenoids are attached to the. As a result few differences in mechanical properties have been.

Low Molecular Weight Thiols In Thiol Disulfide Exchange Antioxidants Redox Signaling
Lutensol XP 89 is a liquid nonionic surfactant 85 activity.

Thiol molar mass 89. 31799-71-0 Suppliers German manufacturers and suppliers of lutensol from around the world. Most crosslinked LCEs are soft rubbery materials at RT a result of their low degree of crosslinking. 47 C 100 C.
The Avogadros number is 6022 X 1023. Sometimes B 2 H 6 is written which is another form. N N N N-tetramethyl-6-carboxyrhodamine.
Lutensol AO 3109 is a mixture of Lutensol AO 3 and Lutensol AO 10 with an active content of ca. Mole - chemical mass unit equal to 6022 x 10 23 molecules atoms or. We would like to show you a description here but the site wont allow us.
Initiator and 246-tris. We thus treated recombinant Nsp9 COV19 with oridonin before gel filtration to remove soluble drug and then digested the eluted protein with trypsin without reduction or alkylation of Cys residues. Evidence for modification.
9451 vvv RT 5 min. Determine the molecular formula of cadaverine. Benzene is an organic chemical compound with the molecular formula C 6 H 6The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each.
Protein farnesylation is a post-translational modification where a 15-carbon farnesyl isoprenoid is appended to the C-terminal end of a protein by farnesyltransferase FTase. 127 C 423 Adjusting Mechanical Properties of the Print. There is the answer.
Intact mass spectrometry MS data were obtained for both free RPE and purified RPE-Ru n. There are three Cys residues within the sequence of Nsp9. It is 5877 C 1381 H and 2742 N.
Q24 Polychlorinated biphenyls PCBs. CH 3 CH 2 OH. Thus whereas the hydrocarbons are insoluble in water alcohols.
It also acts as an adhesion promoter. Molar Absorptivity at 260nm. The molar extinction coefficient of a protein can be estimated by knowing its amino acid composition.
Under green irradiation which corresponds to the maximum of the RPE absorbance 2 was generated in 10 yield with Rubpy 3 2 alone and with the unconjugated Ru. Phenyl methanal into benzoin. To develop the resin-based matrix ELO was crosslinked with dodecenyl succinic anhydride DDSA.
Compared with the control group the 100 nmolL 10 umolL and 1 mmolL BPA groups showed no remarkable changes in the proliferation of Sertoli cells 98 - 8 96 - 3 and 95 - 3 P 005 but the 10 umolL and 1 mmolL of BPA groups exhibited significantly decreased concentrations of intracellular glucose 389 - 007 vs 336 - 024 and 304 - 021 pmolcell P. 4 Eye Dam. 1 pound is 45359 g 45359 g 10 pounds.
90 with the reaction being initiated by an equal mixture of NN-dimethylbenzylamine BDMA. 400 Kilograms Min Order 2 YRS Shanghai Jiujie Mar. Cystine is the oxidized dimer form of the amino acid cysteine and has the formula SCH 2 CHNH 2CO 2 H 2It is a white solid that is slightly soluble in water.
This product appears as a yellow liquid with a viscosity of 1200 Pas a functionality of 55 epoxides per triglyceride and an average mass of 980 gmol 1. Convert 10 lbs to grams divide by the molecular weight of sugar multiply by avogadros number multiply by 12. Molar heat capacity - heat energy required to raise the temperature of 1 mole of a substance 1 Kelvin.
1 Aquatic Chronic 2 H302 H318 H317 H411 015-162-00-8 vanadiumIV oxide hydrogen phosphate hemihydrate lithium zinc molybdenum iron and chlorine-doped 407-350-7 Acute Tox. Conversions for reactions performed on different scales are. It serves two biological functions.
Particularly the mechanism of thiol-maleimide reaction was regarded as a typical Michael addition therefore the reaction rate could be well modulated by altering the pH of aqueous solutions. Proteins encompassing post-translational modifications PTMs such as disulfide bonds and glycosylations present challenges to HDX-MS as disulfide bond reduction and deglycosylation is often required to extract HDX information from regions. Mass of one mole of a substance.
By using the following equation the native protein extinction coefficient in water can be computed using the molar extinction coefficient values at a given wavelength of tyrosine tryptophan and cystine at 280 nm the extinction value of Tyr is 1490 of Trp is 5500 and of Cys is 125 in water. Its molar mass is 102 gmol. Hydrogendeuterium exchange mass spectrometry HDX-MS is a recognized method to study protein conformational dynamics and interactions.
Actinium - the name for the element with atomic number 89 and is represented by the symbol Ac. Alcohols can also engage in hydrogen bonding with water molecules Figure 23 Hydrogen Bonding between Methanol Molecules and Water Molecules. I H2O ii CO2 iii CH4 Question 1.
Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. This modification typically causes proteins to associate with the membrane and allows them to participate in signaling pathways. 31799-71-0 Skype LUTENSOL FSA 10 CAS No.
1 Calculate the molecular mass of the following. Eventually the LCE reaches a temperature at which the strength of the. Because it contains only carbon and hydrogen atoms benzene is classed as a hydrocarbon.
The reaction rate constant at pH 434 was 1000 times higher than that at pH 073 demonstrating the good feasibility in the kinetic control over polymerization reaction. As in low-molar-mass liquid crystals heat introduces kinetic energy to the molecules. C 3 H 8.
Ru n afforded product 2 in 89 yield under red-light irradiation whereas no product formation was observed with Rubpy 3 2. 10 min incubation with a twofold molar excess. Figure 1 Native mass spectrometry of SARS-CoV-2 Nsp9.
KHCO 3 40 µmol DMSOMTBEH 2 O 1 ml approx. Sugar is C12 H22 O11 the molecular weight or molar mass is 342 gmols. Molar volume - volume of one mole of a substance.
4 7 2 4 5 7-Hexachloro-6-carboxyfluorescein. It is a member of the actinide group. 2b and FSO 2 N 3 in 11 molar ratio.
Pt 4 PdBaSO4 quinoline 28. The thiol-ene reaction 1 the hydrothiolation of a C C bond has recently attracted significant attention in the materials arena 2 because it displays many of the attributes of click chemistry. 1 Aquatic Chronic 2 H332 H373 H318 H411 015-163-00-3 bis26-dimethoxybenzoyl-244-trimethylpentylphosphinoxide 412-010-6 145052-34-2.
A site of redox reactions and a mechanical linkage that allows proteins to retain their three-dimensional structure. CH 3 CH 2 CH 2 OH. Molar Absorptivity at 260nm.
4 STOT RE 2 Eye Dam.
Your assessment is very important for improving the workof artificial intelligence which forms the content of this project. River North is packed with Chicagos best bars and clubs but.

Titanium Tetrachloride Wikipedia
Draw Lewis structures for the fluoroethene molecule C2H3F the acetonitrile molecule CH3CN and the acetylene molecule C2H2 in the window below and then answer the questions that follow based on your drawings.

Ticl4 molar mass. The temperature of one of the bulbs is then raised to T2. Weight is the force of gravity on an object. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
In photosynthesis plants convert. C2_A_QUI_ALICE_PROF 051010 1002 Página I Química QUÍMICA A 3a S Curso Extensivo A a 3. Polyethylene was essayed to obtain at higher temperatures and also with lower molar mass polyethylene.
From which of the following compounds can HO- remove a proton in a reaction that favors. PE and PP differ in the relative numbers of these CH bonds. It is directly proportional to mass as shown in the following mathematical expressions.
Tio2 E 171 Rutile Tio2 Titania Titanium Oxide Titanium Peroxide TiO2 Molar Mass TiO2 Oxidation Number. Water - H 2 O. Y on treatment with ethanol in presence of H2SO4 gives a pleasant smelling compound Z which should be an ester RCOOC2 H5 of molecular mass 88.
Although 3 CH bonds are the most reactive the abundance of 2 and 1 CH bonds in PE and PP respectively increases the probability of reaction at these sites. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. In cyclic structures the double bond is always assumed to be on the first carbon.
Use the given densities to convert from volume to mass. TiCl4 Molar Mass TiCl4 Oxidation Number. In chemistry the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula then adding all of these products together.
To write the formula for Copper II nitride well use the Periodic Table and fTypes of Chemical Reactions Synthesis combination reactions - two or more substances combine to form a single substance. Mass Mass describes the quantity of matter in an object. A common mistake is to forget the subscript 2 outside the parentheses in NH42CO3 which could give a much lower molar mass.
Ch3ch2nh2 lewis dot structure. Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chlorideIt is sometimes referred to as tickle or tickle 4 due to the. After identifying the limiting reactant use mole ratios based on the number of moles of limiting reactant to determine the number of moles of product.
Get help with your Chemical bond homework. Exercícios Resolvidos De Química - ID5c1837aec6758. 4 TiSO 4 2 titaniumIV sulfate.
Chemical Bond Questions and Answers. Thus the R will be CH3 mol. Titanium tetrachloride is the inorganic compound with the formula TiCl 4It is an important intermediate in the production of titanium metal and the pigment titanium dioxideTiCl 4 is a volatile liquid.
We may offer the following speculative explanation for these results. 3 BaO 4. Avogadros number and molar mass To convert from grams to number of molecules you must first convert the grams into moles which requires the.
3 CH bonds in PP is 321. W r m and W. More information on molar mass and molecular weight.
Essays with this precatalyst were done in order to know the conditions of ethylene polymerization. Converting amu to grams. Determine the correct formula for titaniumIV chloride.
Now the molecular mass of COOC2 H5 is 72 thus the molecular mass of R will be 15 ie 88 72 16. Grams mole molar mass. Sicl4 structure - ddkzonexpl.
Here we have used the Henderson-Hasselbalch to calculate the A. The final pressure pf is. Book a Party Best Bars in Chicago The best bars in Chicago are not hard to find when you hit up River North.
Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. Hoh Aqua Oh2 H₂O Oxidane Pure Water Hydroxic Acid Hydrogen Oxide H2O Molar Mass H2O Oxidation Number. Access the answers to hundreds of Chemical bond questions that are explained in a.
The molar mass of gaseous molecule is Main Online April 9 2017 28 g mol1 56 g mol1 112 g mol1 224 g mol1 Two closed bulbs of equal volume V containing an ideal gas initially at pressure pi and temperature T1 are connected through a narrow tube wwwjeebooksin of negligible volume. Molar mass did not increase with conversion and in addition to PPh 3 only protonated products were detected by 31 P NMR. Academiaedu is a platform for academics to share research papers.
Thank you for your participation. 11 - The Second Law Jul 04 2020 Correct answers. First fast protonation takes place followed by slow nucleophilic addition of the TfO counterion to the protonated monomer.
Molar Mass of C8H18 1143 g C8H18 Molar Mass of CO2 4401 g CO2 2 mol C818 16 mol CO2 1 mol C8H18 1143 g C8H18 1 mol CO2 4401 g CO2 50 X 102 g C8H118 X 1 mol C8H181143 g C8H18 4374 mol C8H18 4374 mol C8H18 X 16 mol CO22 mol C8H18 3499 mol CO2 3499 mol CO2 X 4401 g CO21 mol CO2 154016 g CO2 154 X 103 g CO2. Thesis scopes The BIQFeCl 2 precatalyst was synthesized and analyzed. More commonly we use the unit gram g about the mass of three aspirin tablets.
Using mole ratios determine which substance is the limiting reactant. In SI the standard of mass is 1 kilogram kg which is a fairly large unit for most applications in chemistry. Titanium Dioxide - TiO 2.
Hydrogen Chloride - HCl. Since the mass is less than half the molar mass 4296 05 the number of formula units should be less than half Avogadros number 26 x I02360 x 1023 05. Then use each molar mass to convert from mass to moles.
Mass spectrometry Section 11. CH3CH2NH2 Malonic Acid ethylene glycol isopropyl alcohol hydrogen bromide SeCl4. Sp2 1 point is earned for the correct answer.
Hcl HCl Molar Mass Bond Polarity HCl Oxidation Number. Four chloride ions would be needed to balance the charge of the titanium ion. Série Ensino Médio C2_A_Q.
The polyethylene obtained with BIQFeCl 2. TiCl4 The IV in titaniumIV chloride indicates that the titanium ion has a 4 charge. PEs comprise a majority of 2 CHs with smaller quantities of 1 and 3 CH bonds while the molar ratio of 1.
The acyloxygen ring opening gives low concentration of a unimer with. Chloride has a -1 charge. Lewis dot Structure is a very simplified representation of the valence shell electrons in a molecule question_answer Q.
Open metalorganic frameworks are widely regarded as promising materials for applications123456789101112131415 in catalysis separation gas storage.