If it is insoluble in water take sodium carbonate extract and add a few drops of sodium nitroprusside solution. Sulphide S 2- Take the water extract and add sodium nitroprusside to it.
Lead Sulphide Precipitate 2 Of 3 Stock Image C036 3892 Science Photo Library
Shipped as a liquid confined under its own vapor pressure.
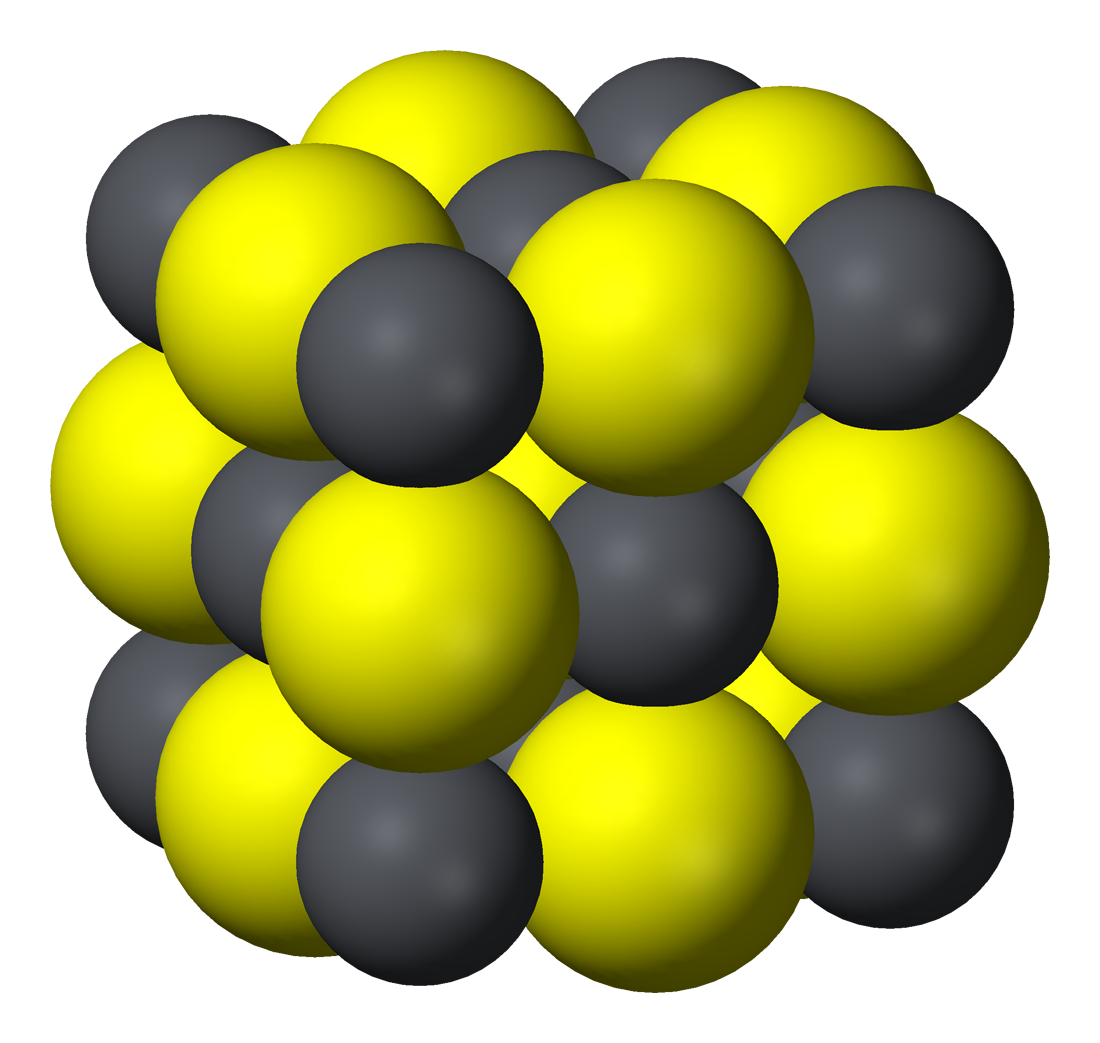
Lead sulphide salt. K sp is the product ot the concentrations of the salt constituent ions each raised to its stoichiometric coefficient. The main structure is the Salt Creek Shear which trends north-south and deforms the belt on a regional scale. For confirmation read todays ICG ann of outcrops up to hundred m long of Cu5 and AG1000 gt see Fig 3 of their ann today - but the key issue is that our turn could come at any time.
The treatment or elimination of pollution from unregulated sources. This is done by conducting a series of tests in a systematic manner and using the observations to confirm the absence or presence of specific cations and anions. 89134 anhydrous basis Compare Product No.
B Print A thin strip of type metal used to separate lines of type in printing. And X denotes a halide I Br or Cl Corner-shared PbX 6 octahedra form a cuboctahedral cage to accommodate the A cation that satisfies the steric requirements and. C Sheets or plates of lead used as a covering for roofs.
Empirical Formula Hill Notation. Lead chloride is a crystalline salt whose formation is encouraged by micro holes in the wall larger surface. Take a salt solution and add cobalt nitrate to it.
Since w e are performing a semi. Group Precipitation cold Pb2 2Cl- PbCl 2 in group l Pb2 S2-H PbS. Galena is the principal ore and the most important compound of lead.
We must also ensure a quantitative precipitation. So we may not get the required clear original solution. An article made of lead or an alloy of lead.
Salt analysis is an integral part of the CBSE Class 12 Chemistry. Its preparation is an entertaining and popular. Purple or violet a Take 01 g of salt in a china dish.
Is iron sulphide compounds. 90 HPLC Expand. The corrosion data in this section is mainly based on the results of general corrosion laboratory tests which are not strictly comparable with actual service conditionsThe corrosion tables provide an initial guide to the selection of materials and are intended to facilitate understanding of the different types of corrosion damage that can arise due to poor material selection.
Adult intake of kitchen salt is on average 9 g per. Restacked two-dimensional hexagonal-MoO3 exhibits high pseudocapacitive. Add 1 mL of ethanol and 02 mL conc.
Formation basic properties related materials. No because sulphuric acid H 2 SO 4 will produce sulphates of the cations and some sulphates like lead sulphate PbSO 4 are insoluble. Salt analysis also known as systematic qualitative analysis or qualitative inorganic analysis involves the identification of the cation and anion of an inorganic salt.
At room temperature it is a bright yellow odorless crystalline solid that becomes orange and red when heated. Purple or violet a Take 01 g of salt in a china dish. A A plummet or mass of lead used in sounding at sea.
All could be lost but if Ayra gives us a long intercept of copper or silver lead both strong in the Mt Isa area re Mt Isa itself and Cannington we are off to the bank with a fist full of dollars. 1 Copper oxide and ferrous sulphide 2 Copper sulphide and ferrous oxide 3 Copper sulphide and ferrous sulphide 4 Copper oxide and ferrous oxide 62. Also add a small amount of lead acetateaq to this.
Sodium shortages may lead to dehydration convulsion muscle paralysis decreased growth and general numbness. Hydrogen sulfide appears as a colorless gas having a strong odor of rotten eggs. In the extraction of lead by self reduction.
The planar growth is hypothesized to occur via a match between the crystal lattices of the salt and the growing oxide. Methylmalonyl coenzyme A tetralithium salt hydrate. Explain how Aluminium III Al 3 can be confirmed using cobalt nitrate.
C 25 H 36 Li 4 N 7 O 19 P 3 S xH 2 O. The lead halide perovskite structure is represented by the general chemical formula APbX 3 where A denotes an organic ammonium or inorganic cation such as methylammonium MA formamidinium FA or Cs. Crop fertilization manure application road deicing salt storage areas unlicensed and closed landfills leaks spills and decommissioning clean-up.
It was formerly called plumbous iodide. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Add 1 mL of ethanol and 02 mL conc.
Lead sulphide Black precipitate b If the salt is soluble in water take the solution of salt in water make it alkaline with ammonium hydroxide and add sodium nitroprusside solution. LeadII iodide or lead iodide is a salt with the formula PbI 2. Inorganic minerals metals and compounds such as sodium copper lead and zinc are common in wastewater from both sewage and wastewater.
If it is insoluble in water take sodium carbonate extract and add a few drops of sodium nitroprusside solution. A purple or violet solution is obtained. Generally humans require about 300 mg sodium chloride per day to warrant a balanced sodium level.
MM-CoA α-Methylmalonyl coenzyme A tetralithium salt. The registry of restriction intentions until outcome lists the intentions and Annex XV restriction proposals received by ECHA. They include non-point source activities ie.
It is chiefly obtained from the mineral galena lead sulphide. Density liquid 83 lb gal. The compound currently has a few specialized applications such as the manufacture of solar cells and X-ray and gamma-ray detectors.
Cross-cutting northeast and northwest faults are secondary with Riedal structures occurring between parallel faults. Contact with the unconfined liquid can cause frostbite by evaporative cooling. 1 Smelting 2 Aluminothermy 3 hydrothermy 4 no specific name 63.
LeadII sulfide also spelled sulphide is an inorganic compound with the formula Pb S. Ag Cl - 10-10 K sp When Ag Cl - 10-10 we get a precipitate. Most inorganic substances are stable and cannot be broken down easily by organisms in wastewater.
It is a semiconducting material with niche uses. LeadII ion Pb2 its coordination number is 3 or 4 its oxide is amphoteric dissolves in both acid and alkali Reactions Important in the Separation and identification of Lead Pb2 1. A chemical substance consisting of two atoms of oxygen combined with one atom of another.
Managing director Brett Wallace said drilling would allow the company to target quartzsulphide veinhosted coppergold mineralisation. The process in which metal oxide is reduced to metal by Al is called. They can originate from industrial and commercial sources stormwater and inflow and infiltration from cracked pipes.
Hence pl a roof covered with lead sheets or terne plates. A black precipitate is formed. Lead sulphide Black precipitate b If the salt is soluble in water take the solution of salt in water make it alkaline with ammonium hydroxide and add sodium nitroprusside solution.
Burn a filter paper which. A restriction proposal may be prepared by a Member State or by ECHA at the request of the Commission or on its own initiative for substances in the Authorisation List. H 2 SO.
Multi-media considerations may lead to more stringent limits compared to those needed to protect the water resource alone. People that have diarrhoea or other health effects that increase salt requirements need a higher dietary amount of sodium than usual. Addition of hydrogen sulfide or sulfide salts to a solution containing a lead salt such as PbCl 2 gives a black precipitate of lead sulfide.
