The 3-D structure of the protein determines which surface residues will be available to contact the columns stationary phase. Propionaldehyde is made by hydroformylation ethylene.
1 Propanol N Propyl Alcohol Ar 201072500 Reagents 71 23 8 Biochemopharma
The complete combustion of methanol is as follows.

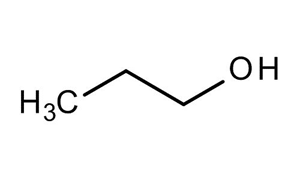
N-propanol structure. IUPAC Name propan-1-ol. These alcohols form hydrogen bond with water due to the polar OH functional group. Concentrated alcohols mainly ethanol 1-propanol called also n-propanol and 2-propanol called isopropanol and mixtures thereof.
2 4 6 Alcohol-free products are generally based on disinfectants such as benzalkonium chloride BAC or on antimicrobial agents. It is a short-chain primary fatty alcohol and a member of propan-1-ols. CATEGORY 1A Sampling and Measurement of noise illumination and heat.
Sample pI is a guideline and not absolute. Oxidation of Propanol CH 3 CH 2 CH 2 OH with PCC and KMnO 4. Propan-1-ol is the parent member of the class of propan-1-ols that is propane in which a hydrogen of one of the methyl groups is replaced by a hydroxy group.
The higher-order alcohol n-propanol is desired for its high volumetric energy density 27 MJ l 1 ref. 1 6 At those concentrations alcohol immediately denatures proteins effectively neutralizing certain types of microorganisms. The net charge determines the form of IEC anion exchange or cation exchange to be applied.
This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. 1-Propanol is a primary alcohol with the formula CH 3 CH 2 CH 2 OH and sometimes represented as PrOH or n-PrOHIt is a colorless liquid and an isomer of 2-propanolIt is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry mainly for resins and cellulose esters and sometimes as a disinfecting agent. As the number of carbons per polar functional group increase solubility decreases.
Valid until September 27 2022. Prices Kandla Mumbai Mundhra Markets covered. Propan-1-ol n-propyl alcohol 1-propyl alcohol or n-propanol are all names for this colourless oil.
Keeping in mind the relative molecular weights of the compounds you can see there is a decreasing effect of the hydrogen bonding and other effects on the n-alcohol series as we move to larger chains. Further 2-phenoxyethanol and 1- and 2-phenoxypropanols are used phenolic substances such as phenol also called carbolic acid cresols such as thymol halogenated chlorinated brominated phenols such as hexachlorophene triclosan trichlorophenol. Please note that Hydrophilic PVDF syringe filters are not compatible with majority of strong acids and caustic solutions such as dimethyl sulfoxide dimethylformamide DMF acetone ketones esters and ethers.
Our PVDF filters are high defined pore structure low nonspecific binding compatible with organic and aqueous solvents. Propionaldehyde is catalytically hydrogenated to produce 1-propanol. NSC 68472 Permanent link for this species.
1-Phenyl-1-propanol C9H12O CID 7147 - structure chemical names physical and chemical properties classification patents literature biological activities. 2CH3OHl3O2g2CO2g4H2Ol Which of the following statements is true regarding the. Alcohol-based products typically contain between 60 and 95 percent alcohol usually in the form of ethanol isopropanol or n-propanol.
Ethyl and isopropyl alcohol are similar molecularly but have different chemical structures. The surface structure of the copper electrodes strongly depends on the pre-treatment 35 and reconstructions take place under the CO 2 RR 363738Figure 1 and. The distinct lattice fringe of 0270 nm corresponds to the 221 crystallographic plane of GaFeO 3 phase.
Which of the following classes of alcohols will oxidize under mild conditions to form a ketone. Calculate the heats of combustion of these alcohols. Compatible with organic and aqueous samples.
Academiaedu is a platform for academics to share research papers. Also propanol can be oxidized to propanal by mild oxidation agents such as PCC. Data obtained from the NIST Webbook.
Cation exchange is used at pHs below a proteins pI while anion exchange is used at pHs above a proteins pI. When 100 g of each of these alcohols is burned in air brat is liberated as indicated. The solvent properties of isopropyl alcohol is similar to ethyl alcohol.
COMPANY NAME ACCREDITATION NUMBER VALIDITY CATEGORY ADDRESS CONTACT DETAILS. 2i-l displayed the selected SEM image of GaFeO 3 and the EDS elemental mapping images of Ga Fe and O. Propanol 1-propanol n-propanol is a primary alcohol which can be oxidized to propanoic acid by using strong oxidizing agents.
The solubility of alcohols with four to five carbons is given. Organic compounds with one polar functional group and a low number of carbon atoms such as methanol ethanol and n-propanol are highly soluble miscible in water. Fill in the missing atoms or groups to complete the structure of the more complete oxidation of n-propanol below.
H O OH H. Low-density polyethylene LDPE films coated with a mixture of polyamide resin in i-propanoln-propanol and a bacteriocin solution showed an antimicrobial activity against Micrococcus flavus. Propanol is one of the most common types of alcohol.
9cost-effective renewable propanol would offer a sustainable liquid fuel for existing. Isopropyl alcohol is widely employed in solvent applications. The internal loose structure of the hierarchical GaFeO 3 may enable the material desirable gas sensing performance by virtue of excellent gas diffusion and fast mass transportation.
In chemistry the definition of alcohol is an organic molecule that contains a hydroxyl group bonded to. Methanol ethanol and n-propanol are three common alcohols. A must-have and indispensable service for individuals and organizations associated with chemical markets across the.
This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Propanol has the formula CH 3 CH 2 CH 2 OH. We would like to show you a description here but the site wont allow us.
Use this link for bookmarking this species for. The incorporation of 10 ww potassium sorbate in low-density polyethylene films lowered the growth rate and maximum growth of yeast and lengthened the lag period before mould growth Han and Floras. Isopropyl alcohol was one of the first petrochemical products to be manufactured and produced since 1920.
Isopropyl alcohol commonly referred to as Isopropanol or n-propanol or dimethylcarbinol is a colourless and flammable liquid with the formula C 3 H 8 O. It has a role as a protic solvent and a metabolite.
The molal elevation constant is the ratio of the elevation in boiling point to. This value indicates that 2-phenylisopropanol will be essentially nonvolatile from water surfaces2SRC.

Henry S Law Constants For N Alkane Adsorption On Al And Cl Download Scientific Diagram
The Henrys Law constant for nitrogen gas in water at 30 degrees C is 60 times 10-4 Matm.

Henry's law constant value for 2-propanol. KPm0438 where P is given in the atmosphere and m is the molality. ML for the combined volume of CV and NaOH because it. They demonstrated that Henrys law constants decrease with increasing temperature.
Davies and Gilbert 1941. To increase the O 2 concentration in internal fluids organisms synthesize highly soluble carrier molecules that bind O 2 reversibly. Under standard conditions it is also equal to negative R T times the natural log of K where R is the gas law constant T is the absolute temperature and K is the equilibrium constant for the reaction.
Academiaedu is a platform for academics to share research papers. Average of 7 values. Solution is given by.
Osmotic pressure of a dil. Critical compilation correlation and recommended data. A Molarity b Molality c Mole fraction of solute d Mole fraction of solvent 22.
2014 Larger mean free path for X as compared to that of Y Larger. An existing analytical method including a solid-phase extraction and derivatization before GCFID analysis is available but presents some disadvantages. They also presented a modified method based on Peng-Robinson PR EOS.
DAngelo and Francesconi measured the solubility of H 2 in several n-alcohols ie 1-butanol 1-propanol ethanol and methanol. This can only be true in the limit of zero pressure where the molecules of the gas are very far apart. Henrys law constants at 25 C of the studied PFRs vary between 28 10 4 atm-m 3 mole 1 for tri-iso-butyl phosphate TiBP Chemspider 2011 until 17 10 23 atm-m 3 mole 1 for THPS Syrres 2011.
To increase the O 2 concentration in internal fluids organisms synthesize highly soluble carrier molecules that bind O 2 reversibly. 2-Phenylisopropanols vapor pressure 0047 mm Hg3SRC indicates. The value of d in cm shown in the figure as estimated from Grahams law is Adv.
Solutions to exercises 1A1b The perfect. A b c d 12 16 20 The experimental value of d is found to be smaller than the estimate obtained using Grahams law. Δ f H liquid-2259 13.
Solutions of hydrogen chloride in chlorobenzene obeys Henrys Law. Value Units Method Reference Comment. The major metabolites identified in urine were 2-phenyl-2-propanol R-.
Daltons law is a limiting law because it holds exactly only under conditions where the gases have no effect upon each other. A Victor-Mayers method b Grahams law of diffusion c Gay Lussacs law d Raoults law. Quantity Value Units.
Δ c H liquid-4817. Calculate the temperature in C that must be maintained in a gas carrier tank in the form of a horizontal cylinder with. This is due to Adv.
Based on your answer to Questions 35 design an experiment for the reaction of CV with NaOH and describe the subsequent data analysis to accomplish the Central Challenge the determination of the value of i w the order with respect to CV and ii k the pseudo-rate constant found in the rate law in Equation 3. Due to the low Henrys law constant for O 2 in water the levels of dissolved oxygen in water are too low to support the energy needs of multicellular organisms including humans. What is the partial pressure of HCl in mmHg over a 1 by weight solution of HCl in chlorobenzene.
FTP identify this quantity named for a Yale chemist which is a measure of the spontaneity of a reaction. Hence Daltons law holds exactly only for a mixture of perfect gases. For example human red blood cells contain a protein called hemoglobin that.
If the N_2 gas is present at 15 atm what is the solubility of N_2. 2014 a 8 wwwjeebooksin b c d 49. Quantity Value Units Method Reference Comment.
1-Methoxy-2-propanol 1M2P is one of the dominant glycol ethers and the unmetabolized urinary fraction has been identified to be a good biological indicator of exposure. Thus a high negative value indicates a forward-going reaction. Temperature dependences of limiting activity coefficients Henrys law constants and derivative infinite dilution properties of lower C-1-C-5 1-alkanols in water.
Handbook of Chemistry and Physics 84th - David R Lide. For real gases the law is only an approximation. The wide range of Henrys law constant values of PFRs indicates that the distribution of PFRs over air and environmental waters like the oceans is highly variable.
They calculated Henrys law constants and proposed simple empirical correlations for solubility data. Molecular weight of non-volatile solute can be determined by. If the N_2 gas is present at 15.
Due to the low Henrys law constant for O 2 in water the levels of dissolved oxygen in water are too low to support the energy needs of multicellular organisms including humans. Δ f H liquid-2244 079. For simplicity use 10.
For example human red blood cells contain a protein called hemoglobin that. Prosen and Rossini 1945. The Henrys Law constant for 2-phenylisopropanol is estimated as 38X10-7 atm-cu mmoleSRC using a fragment constant estimation method1.
We present here an alternative method for the determination of urinary.