Argon - Density and Specific Weight - Online calculator figures and tables showing density and specific weight of argon Ar at varying temperature and pressure - Imperial and SI Units. Gases A and B each exert 220 mmHg.
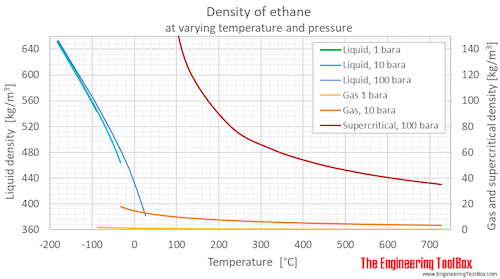
Ethane Density And Specific Weight
The pressure rises with LPG temperature.
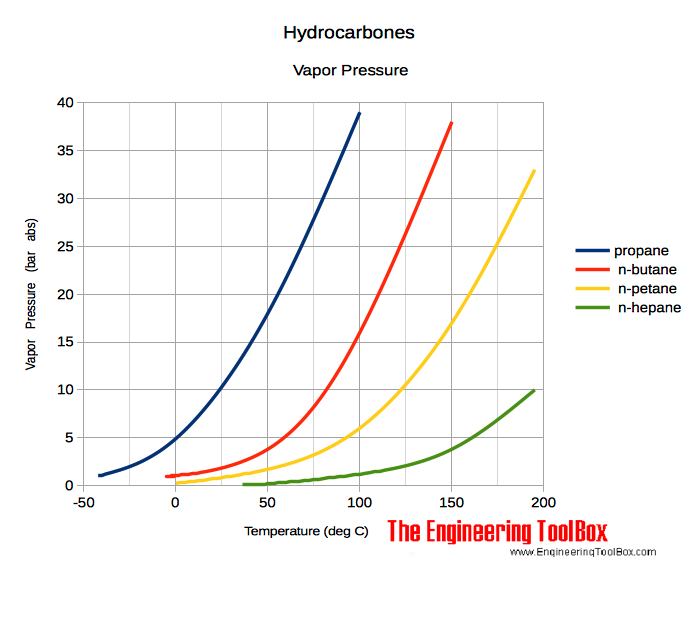
Vapour pressure ethane. Flaring processes can be classified into three. For example methylbenzene is converted by. NGLs include propane butane isobutane ethane ethene propene isobutene butadiene pentane and pentene and pentanes plus.
Key considerations for design and operation of a renewable diesel unit White Paper. Hold eyelids apart for 15 minutes. It generates 0 kPa at -43C but at 38C 100F it generates 1186kPa or 172 PSIG.
This percentage increases as the temperature goes up. LPG gas pressure varies with temperature. Iodoethane is an iodoalkane that is ethane substituted by an iodo group.
The Vapour Pressures of Pure Substances. Density and specific weight Dynamic and kinematic. Pentanes Plus is a mixture of liquid hydrocarbons mostly pentanes and heavier.
As mentioned before LPG is stored in a gas bottle under pressure. 500 gL at 20 C OECD 105 EC A6 shake flask method f. Ethylenediamine appears as a clear colorless liquid with an ammonia-like odor.
If the liquid is heated a little over 100 C the transition from liquid to gas will occur not only at the surface but. The CAMS reanalysis was produced using 4DVar data assimilation in CY42R1 of ECMWFs Integrated Forecast System IFS with 60 hybrid sigmapressure model levels in the vertical with the top level at 01 hPa. Immediately flush with tepid water or with sterile saline solution.
At 100 C and atmospheric pressure equilibrium is not reached until the air is 100 water. Vapor pressure or vapour pressure in British English. Ammonia - Vapour Pressure at Gas-Liquid Equilibrium - Figures and table with ammonia saturation pressure at boiling points SI and Imperial units.
The vapour pressure of ammonia is the pressure at which ammonia gas is in thermodynamic equilibrium with its condensed state. See spelling differences or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases solid or liquid at a given temperature in a closed systemThe equilibrium vapor pressure is an indication of a liquids evaporation rate. A sample of argon gas is.
For example the windows of the capsules are made of reinforced glass using polyvinyl butyral PVB as the laminate which is anchored to the metal frame with a silicone resin. Atmospheric data are available on these levels and they are also interpolated to 25 pressure 10 potential temperature and 1 potential vorticity levels. Gas generates mainly water vapour and CO 2.
Ethane 74-84. Flash point of 91F and a melting point of 47F. Stephenson and Malanowski 1987.
What is the total pressure of the mixture inside the flask. Assuming no enthalpy of mixing for vaporised ethanol and hydrocarbons then the enthalpy of liquid mixing will lead to a corresponding reduction in the enthalpy of vaporisation. Raw natural gas also contains impurities including water vapour hydrogen sulphide H2S carbon dioxide helium nitrogen.
Vacuum distillation allows heavier fractions to be further separated without high temperatures which could break them down. 3 Chemical and Physical Properties Expand this section. Cicotti 1992 A47879 Henrys Law constant KH 448 10-9 Pa m3mole calculated Schollmeier 1992 A48583 Solubility in water including effect of pH water at pH 54 7 89.
25ppm Alternative Names UN Number Propane LP Gas or LPG Butane 1978 1075 1011 4 First Aid 41 Description of first aid measures Eye Cold burns. Lowering the pressure over a liquid will lower its boiling point. Ta TECQUIPMENT PRESSURE-ENTHALPY CHART R-1341 1112-TETRAFLUOROETHANE 500 700 600 400 300 100 200 Grant Peop3b for Icona 17 bar Tg.
The term pressure refers to the average force per unit of. For a 20 volumetric ethanol mixture this means that the enthalpy of vaporisation is about 15 lower than that predicted by a. What pressure is exerted by gas D.
Why a higher shaker speed improves the accuracy of measurement. Disproportionation is said to occur when a reactant is transformed into two or more dissimilar products. At room temperature and pressure the water jar reaches equilibrium when the air over the water has a humidity of about 3.
17 T2-86 Tg 582 t Tuss004 10 100 his ugg bilky houso holky horas lastly SON quid Wie Supech Vapour Para 10 Pressure Bar Pressure MPa PE 30 DO 00 01 10 AN 15 20 30 49 01 001 700 100 200 300 400. The pressure at which LPG transitions between liquid and vapour is called its vapour pressure. Gas C exerts 110 mmHg.
Surface or single level. At higher pressures ammonia would condense. Vapour pressure Estimated to be 31 10-5 Pa at 50 C OECD 104 EC A4 c.
Low pressure pipe flares are not intended to handle liquids and do not perform effi-ciently when hydrocarbon liquids are released into the flare system 10. Figures 2 and 3 Silicones played an important part in the construction of the London Eye the largest observation wheel in the world. Troubleshooting and hydraulic analysis identified vapour channelling in fixed valve trays as the root cause of premature foam-flooding in a high pressure.
A 150 liter flask at a temperature of 25C contains a mixture of 158 moles of methane 09 moles of ethane and 044 moles of butane. Selected Values of the Temperature Dependence of the Vapour Pressures of Some Pure Substances in the Normal and Low Pressure Region 2nd ed Elsevier New York 1984 972. 1 Structures Expand this section.
Natural gasoline is the largest component of pentanes plus. Petroleum is a mixture consisting mainly of alkane hydrocarbons Petroleum fraction. See also properties of Ammonia at varying temperature and pressure.
This resin is prepared in situ from two components one of which is a silicone with. Vapors are heavier than air. It relates to the tendency of particles to.
Premature foam-flood in an amine absorber. Methylbenzene vapour and hydrogen are passed over a catalyst of chromium platinum or molybdenum supported on silica or aluminium oxide at 820-920 K at 40-60 atm pressure. A equal amount of benzene and toluene as it forms an ideal solution b unequal amount of benzene and toluene as it forms a non ideal solution c higher percentage of benzene d higher percentage of toluene 6Which of the following is the reason for Zinc not exhibiting variable oxidation state a inert pair effect b.
Vapour of benzene 128kPa and vapour pressure of toluene 385 kPa. This has also been inferred from vapour pressure measurements by Kretschmer et al. Crude oil vapour pressure testing.
2 Names and Identifiers Expand this section. 1370 gL at pH 5 22 gC in-house method Goerlitz 1990 A43735. At this equilibrium condition the vapor pressure is the saturation pressure.
Ethyleneethane separation is a critical process in the petrochemical industry giving a worldwide ethylene production exceeding 150 million metric tons in 2016. A mixture of four gases exerts a total pressure of 860 mmHg. Efficient combustion in the flame depends on achieving good mixing between the fuel gas and air or steam 9 and on the absence of liquids.
Produces toxic oxides of nitrogen during combustion. It derives from a hydride of an ethane.