What is the symbol for palladium. Elemental hydrogen H element 1 nitrogen N element 7 oxygen O element 8 fluorine F element 9 and chlorine Cl element 17 are all gases at room temperature and are found as diatomic molecules H2 N2 O2 F2 Cl2.

Pin By Richard Vaughn On Chemistry Chemistry Education High School Chemistry Sixth Grade Science
The gaseous element group.

Periodic table gases at room temperature. Ppt The Periodic Table Of Elements Powerpoint Presentation Free. At 0C the elements in their gaseous form are Hydrogen Helium Nitrogen Oxygen Fluorine Neon Chlorine Argon Krypton Xenon and Radon. We can use this Periodic Table of the Elements and find that at a given temperature the elements that are in red are in their gaseous stage.
Hydrogen oxygen nitrogen neon argon helium radon. Hart gave you 118 2. __8762 amu or g_____ 5.
How many elements are gaseous at room 24 periodic table gases at room element is liquid at room temperature classification of the elements. C The freezing point of chlorine is -101C. Click any element below to see all the samples of that element.
How many elements are listed in the periodic table. Elements That Are Liquid At Room Temperature. In the periodic table group 17 Halogen family have metals non-metals liquid and gas at room temperature.
The whole group contains gases those are helium He neon Ne argon Ar krypton Kr xenon Xe and radon Rn. Click here to buy a book photographic periodic table poster card deck or 3D print based on the images you see here. What is the atomic number of selenium.
The noble gases are found in group 18 and exist as isolated atoms. There are a total of 11 elements in the periodic table which are gases at room temperature and standard atmospheric pressure. Iodine is a metal whereas chlorine and bromine are the non-metals.
They are all stable because their outer energy level is filled. The elements that are gases at room temperature are radon Rn xenon Xe krypton Kr argon Ar chlorine Cl neon Ne fluorine F oxygen O nitrogen N helium He and hydrogen H. B The density of chlorine gas at standard temperature and pressure is 317 gL.
Hydrogen H nitogen N oxygen O fluorine F chlorine Cl and noble gases helium He neon Ne argon Ar krypton Kr xenon Xe radon Rn are gases at standard temperature and pressure STP. Fluorides are present in liquid state while chlorine bromine and gaseous are present in the form of gases. Hydrogen H nitrogen N oxygen O fluorine F chlorine Cl helium He neon.
At standard temperature and pressure there are 11 which are gasses. D Chlorine combines with sodium to form table salt. Four other elements are liquids slightly warmer than room temperature.
They are always gases at room temperature. The color of chorine gas is green. They are francium cesium gallium and rubidium all metals.
Written By MacPride Sunday December 23 2018 Add Comment. As the group goes down the density of each atom increases. Considering the periodic table theres first of all one prominent group the noble gases group 8A.
Gases On The Periodic Table At Room Temperature. What is the atomic mass of strontium. Tokyo Electron S Tel Augmented Reality Ar Enhanced.
These elements are gasses at room temperature and pressure. How many elements are gases in the periodic table. D Chlorine combines with sodium to form table salt.
Elemental hydrogen H element 1 nitrogen N element 7 oxygen O element 8 fluorine F element 9 and chlorine Cl element 17 are all gases at room temperature and are found as diatomic molecules H2 N2 O2 F2 Cl2. They do not react with other common elements and do not form bonds in nature. They are colorless odorless and tasteless.
They are mercury a metal and bromine a halogen. Periodic Table Gases At Room Temperature. There are 11 total and you may recognize them if youve looked at different sections of the periodic table which catalogs and organizes chemical elements by their structures and similar properties.
Only two elements on the periodic table are elements at room temperature. Oxygen Hydrogen Nitrogen Fluorine Chlorine and all of the inert gases are gases at room temperature and pressure. 115 rows Elemental hydrogen H element 1 nitrogen N element 7 oxygen O element 8 fluorine.
How are elements that are gases at room temperature designated in this periodic table.
The hydrogen halides are gases at room temperature. Potassium permanganate is standardized against pure oxalic acid.
Hydrochloric acid is also known as muriatic acid and under this name is often sold with swimming-pool supplies.
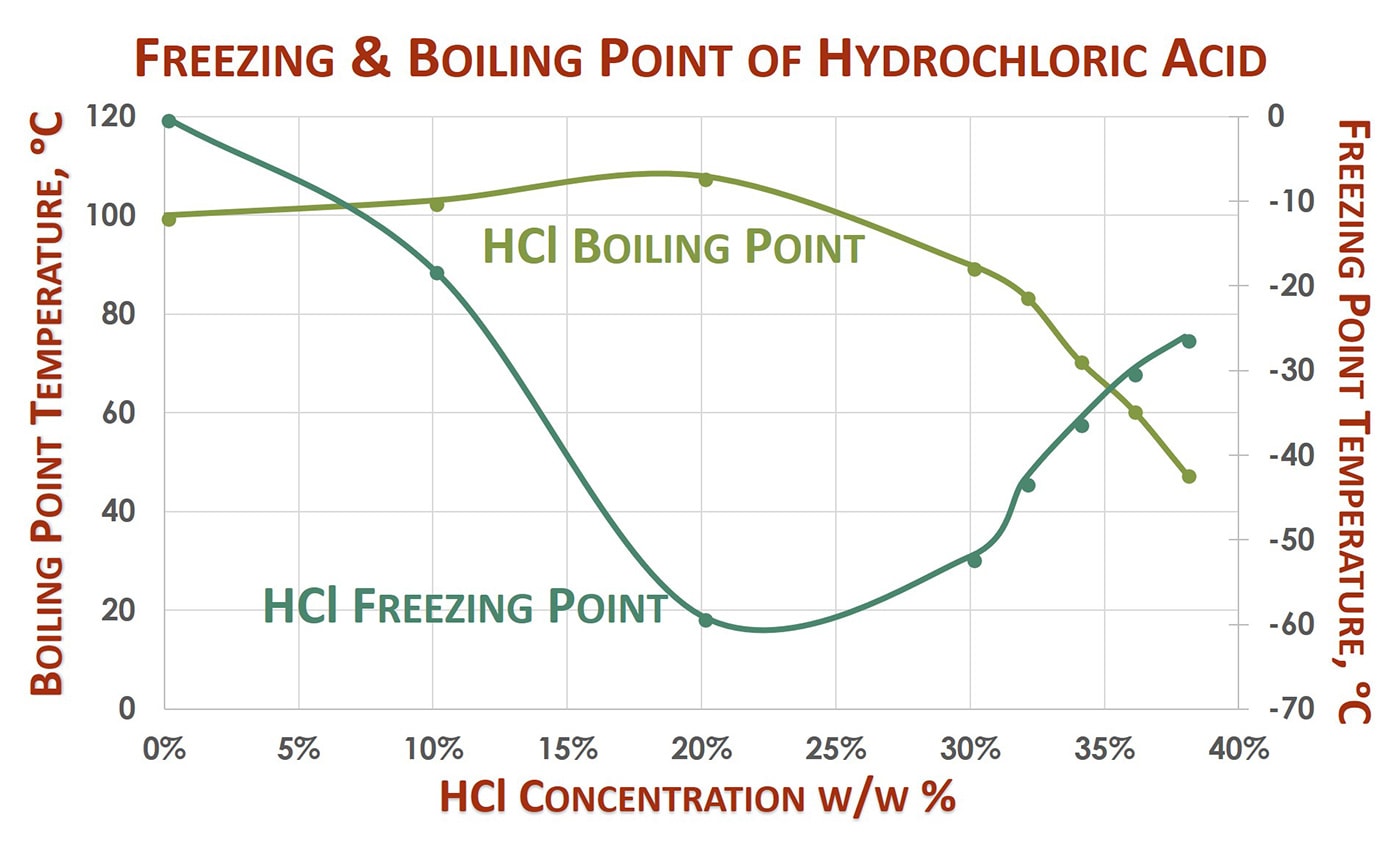
Hydrochloric acid state at room temperature. Oxalic acid is oxidised to carbon dioxide by KMnO 4 which itself gets reduced to MnSO 4. On exposure to air hydrogen chloride forms dense white corrosive vapors. Oxalic acid reacts with potassium permanganate in the following way.
Metal and nometal remember hydrogen can act either way Two nonmetals. The pH of the water solution of sodium benzoate may then be lowered to 10 by addition of hydrochloric acid at which point pure benzoic acid crystallizes and may be isolated by filtration. D Name a common device in which this metal is used.
Hydrogen chloride can be released from volcanoes. We offer free revision as long as the client does not change the instructions that had been previously given. A standard acid-base reaction occurs as sodium bicarbonate aka baking soda is a weak base.
For a discussion of how acidity is influenced by molecular structure Click Here. It has a role as an explosive an antiseptic drug and a fixative. Picric acid is a C-nitro compound comprising phenol having three nitro substtituents at the 2- 4- and 6-positions.
Imidazolium chloride ImCl was prepared in the same way but an excess equimolar of hydrochloric acid the molar ratio of HCl to imidazole was 111 was used in the first step. Arterial blood gases are drawn and the nurse. Hydrogen chloride dissolves in water to produce hydrochloric acid HClaq.
It is used in the synthesis of organochlorine compounds the pickling of steel and other metals to dissolve scale from their surfaces and. An example of a weak acid is vinegar. On exposure to air hydrogen chloride forms dense white corrosive vapors.
The solution is known as bromine water. This doesnt mean that the pure water is becoming acidic or alkaline but that at these temperatures those particular pH numbers represent the neutral point. Phase at Room Temperature.
At room temperature hydrogen chloride is a colorless to slightly yellow corrosive nonflammable gas that is heavier than air and has a strong irritating odor. Aluminum atoms contain 13. The client is hypoventilating and has a respiratory rate of 10 breathsmin.
A client with a 3-day history of nausea and vomiting presents to the emergency department. Answer 1 of 4. In case a client want to alter the instructions revision can be done but at a negotiated fee.
Sodium chloride NaCl Sulfuric Acid H 2 SO 4 Methane CH 4 Hydrochloric acid HCl Chemical Species. Aspirator vacuum pump 1 each 213100 Beaker 400-mL 1 each 50048 Filter membrane 47-mm 08-microns Program 125 1 100pkg 2640800. Like chlorine water it is a good oxidizing agent and it is more useful because it.
Hydrochloric Acid Solution 10 N varies 1 L 2321353 Sodium Hydroxide 100 N varies 1 L 104553 Water deionized varies 4 L 27256 Required apparatus Description Quantitytest Unit Item no. The chemical reaction at. Above and below this temperature it can vary.
This strong acid aids in digestion and kills ingested microbes. For example at 100C the pH of pure water is 614 whilst at 0C its 747. E Can this metal.
B Write the name and formula of the sulphide ore. At the end the residue ImCl was placed in a vacuum oven pressure 6 kPa for 5 h at 80 C and the amount of water by Karl. That is some of their hydrogen ions remain bonded within a compound in solution.
The basicity of oxygen nitrogen sulfur and phosphorus compounds or ions may be treated in an analogous fashion. We give 100 refund for an assignment that we cant complete that had been paid for. Hydrogen chloride has many uses including cleaning pickling electroplating metals tanning leather and.
Also remember to state the exact time the writer should take to do your revision. This process is found in all living. The phosphoryl chlorides are removed by pressing the solid with a spatula on a.
C Give the equations of chemical reactions involved in the production of metal from its sulphide ore. A metal which exists as a liquid at room temperature is obtained by heating its sulphide ore in the presence of air. 270 grams per cm cubed.
The experiments tested yeast respiration in both warm water at 42 degrees Celsius and at room temperature. Like The solution is known as bromine water. Options 1 3 4 are incorrect interpretations.
Strictly speaking pure water only has a pH of 7 at room temperature 25C. As a side note it. Weak acids do not ionize completely.
Hydrogen chloride can be released from volcanoes. About 341 grams 012 ounce of bromine dissolve in 100 millilitres 01 quart of water at room temperature. NaHCO3 aq HCl aq NaCl aq CO.
Will my paper be. Aluminum is the second element in the thirteenth column of the periodic table. Hans Orsted in 1825 first isolated by Friedrich Wohler in 1827.
It is classified as a post-transition metal and a poor metal. After stirring the excess of HCl and water were evaporated with a rotary evaporator. 35-Dinitrobenzoyl chloride is usually partially hydrolysed and should be prepared in the pure state by heating gently a mixture of 35-dinitrobenzoic acid 1 g and phosphorus pentachloride 15 g in a dry test tube until it liquifies 5 min The liquid is poured on a dry watch glass and allowed to solidify.
It derives from a 135-trinitrobenzene and a phenolIt is a conjugate acid of a picrate anion. Concentrated hydrochloric acid is about 37 HCl about 12 molesL. Test your comprehension with this quiz.
Hydrochloric acid HCl which is released from cells in the lining of the stomach is a strong acid because it releases all of its H in the stomachs watery environment. Youll get an aqueous solution of the salt sodium chloride in this case water and carbon dioxide gas given off as bubbles out of the solution. Loss of gastric fluid via nasogastric suction or vomiting causes Metabolic Alkalosis as a result of the loss of hydrochloric acid.
State at Room Temperature. The outcome of the experiment indicates the warm water is optimal for yeast respiration in comparison to cold water. At room temperature hydrogen chloride is a colorless to slightly yellow corrosive nonflammable gas that is heavier than air and has a strong irritating odor.
They dissolve in water to produce acidic solutions. A Name the metal and write its chemical symbol. Respiration is the process that converts sugar known as glucose to energy in this case ATP Adenosine Triphosphate.
Aqueous solutions of hydrogen chloride are known as hydrochloric acid. It involves a redox reaction. The two main types of chemical bonds are ionic and covalent bonds.
Hydrogen chloride has many uses including cleaning pickling electroplating metals tanning leather and.
The concentration of a gas in a solution is directly proportional to pressure. Base your answer to the following question.

The Response Of The Zn 2 Tio 4 Sensors To Propanol At A Concentration Download Scientific Diagram
1 E 9 disintegrationssec 4.
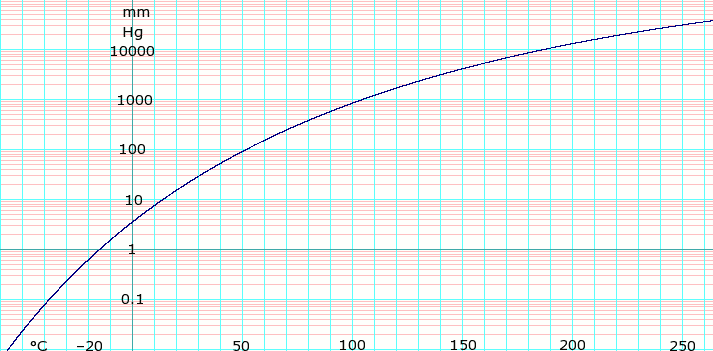
Is propanol a gas at room temp. Na H Cl- and OH-63 Bond Energy This is the amount of energy consumed or liberated when a bond is. Volatile organic compounds VOCs are those organic compounds with a Reid vapor pressure of over 103 Pa at normal temperature 29315 K and pressure 101325 kPa. How do you tell if a compound is solid liquid gas or aqueous.
The concentration of a gas in solution is independent of pressure. Separation Process Principles- Chemical and Biochemical Operations 3rd Edition. Your browser will take you to a Web page URL associated with that DOI name.
Due to the high temp. Anthropogenic CO2 emissions are considered the major contributor of greenhouse gas emissions worldwide. SAFETY Materials Nature 2-methyl-2-propanol 2-chloro-2-methylpropane hydrochloric acid calcium chloride Indicate by signing that you have understood the information in the safety table.
Urine was analyzed immediately 1 2 8 and 9 hr after drinking during 2 hr 375 mlkg of beverages containing orange juice 15 or 40 ethanol and and 1 gl of 1-propanol 2-propanol 1-butanol 2-butanol isobutyl alcohol or a mixture of 1-propanol isobutyl alcohol. Researchers have been focused on developing new formulations of solvents to make more competitive CO2. In cold weather it is therefore solid at room temperature.
If 1 mol of gas is removed from the gas phase what wil. H 2 constant flow H 2 constant flow Flow Rate. Hydrogen is a molecular gas.
Numerous factors including environment temperature exposure time and other conditions can. This means that methanol 148F boiling temp will start to boil before the Determine the pressure of the gas trapped in the apparatus shown below when the atmospheric pressure is 695 mmHg. Easy with positive results as fast as.
I understand the safety information Demonstrators Initials. Aside from looking at. Send questions or comments to doi.
Tests are typically conducted at room temperature. The constitutional isomer of ethanol dimethyl ether CH_3OCH_3 is a gas at room temperature Suggest an explanation for this observation. Please use this book to increase your knowledge for the laboratory pratictioner.
Over time one becomes familiar with certain substances. 1 E 6 disintegrationssec D. A Regulation is the appropriate legal instrument as it imposes clear and detailed rules which do not give room for diverging transposition by Member States.
The gas-sensing response value of the sensing material is defined as S R a R g R a and R g are the resistances of the. Specifically polyethyleneimine PEI grafted ZIF-8 PEI-g-ZIF-8 was synthesized in situ by using Zn2 2-methylimidazole and hyperbranched PEI through a rapid method at room-temp. 1 E 8 disintegrationssec B.
I start my students with learning the standard states of the elements. Basic O-Ring chemical resistance compatibility information is based on isolated generic O-Ring material testing in optimal conditions at room temperature and pressure. Urine was analyzed immediately 1 2 8 and 9 hr after drinking during 2 hr 375 mLkg of beverages containing orange juice 15 or 40 ethanol and 1 gL of 1-propanol 2-propanol 1-butanol 2-butanol isobutyl alcohol or a mixture of 1-propanol isobutyl alcohol.
Moreover a Regulation ensures that legal requirements are implemented at the same time throughout the Community. E26-4 LAB-WORK Physical. 83 C and mp.
Specific material compound formulations can. 1 E 4 disintegrationssec C. Moreover a Regulation ensures that legal requirements are implemented at the same time throughout the Community.
Exposing rubber O-Ring materials to multiple chemicals and compounding application factors like temperature pressure and gland design can result in significantly different performance. If 200 g of helium gas and 400 g of oxygen gas are mixed together what is the mole fraction of helium in the solution. During electrolysis aluminium is produced at the carbon cathode and oxygen at the carbon anode.
The sensor was dried at room temperature for 24 h and then aged at 200 C for 48 h prior to the gas sensing measurements to stabilize the sensing performance. Combined Sensitivity 933 Combined Specificity 988 95 Overall Accuracy Use Whole Blood Plasma or Serum Fast. One Rutherford is equal to A.
A correct statement of Henrys law is. The volume of an ideal gas is zero at _____. A 100 L container contains 10 L of a solution of a gas.
The target gases of desired concentrations were then injected into the test chamber by using a micro-syringe. The VOCs are a large group of carbon-based chemicals that easily evaporate at room temperature Li et al 2009 Ojala et al 2011 Olsen and Nielsen 2001. And porous structure of PEI-g-ZIF-8 were investigated by Fourier transform IR spectroscopy powder X-ray diffraction N2 adsorption-desorption measurement etc.
View Answer You are given a mixture of benzophenone. The mitigation of this kind of CO2 emissions relies on a portfolio of alternatives where CO2 absorption appears as the nearest approach to be applied at industrial scale. 110 539544 1998.
Which of the following will evaporate easily in a dry cloth. The oxygen reacts with the graphite anode to form CO2 and so anode had to be periodically replaced 59 Electrolysis of Brine Brine is concentrated NaCl solution Ions present. 200 ppm is equal to.
A Regulation is the appropriate legal instrument as it imposes clear and detailed rules which do not give room for diverging transposition by Member States. Identity of solute percentage by mass and vapor pressure of pure solvent Vacuum pressure is measured relative to ambient atmospheric pressure. 100 C to 260 C at 35 Cmin hold 025 min 100 C to 260 C at 54 Cmin hold 015 min 100 C to 240 C at 35 Cmin.
The information contained in this chemical compatibility guide is believed to be reliable but has been compiled from numerous industry sources and should be used as a general reference guide only. Assume gas 15 times 10-4 M in solution when 2 mol of gas remain in gas form. Hg and Br are liquid H2 N2 O2 F2 Cl2 and the inert gases are.
Synthesis of vinyl polymers substituted with 2-propanol and acetone and investigation of. Answer 1 of 4. At room temperature and.
FID 250 C. The results indicated that the. Type or paste a DOI name into the text box.
Forms explosive nitrogen trichloride from biuret contaminated with cyanuric acid. By-products include red phosphorus suboxide.

Phosphorus Trichloride P212121 Store
Phosphorus is a waxy white solid and is colorless and transparent when pure.
Phosphorus trichloride at room temperature. 2P 5Cu2 8H 2O 5Cu 2PO 4 3 16H Identify the oxidising agent. Carbon phosphorus sulfur selenium and iodine. Sublimation The transition of a substance directly from the solid to the gas phase without passing through a liquid phase.
HCl hydrogen chloride. Defining the 11-difluoroethane as the system do you expect sys for the process to be positive or negative. One nonmetal in the p-block bromine is a liquid at room temperature.
Only include the outer shell electrons. A white solid that melts at room temperature it is waxy crystalline and highly toxic with garlic odour. Given your answers to parts A B and C do you think the operation of this product depends more on heat flow or more on.
We are forced to write its structure as. Sample form is powder and powder particle is about 40 μm. Molecules such as BeCl 2 and BCl 3 are referred to as electron deficient because some atoms do not have complete octets.
J Phys Chem 106. Relative atomic mass The mass of an atom relative to that of carbon-12. Carbohydrates sugars and starch Lipids fats Nucleic acids DNA and RNA Note that organic compounds are all covalent compounds.
Draw a dot-and-cross diagram for a molecule of phosphorus trichloride. 2 e The equation for the reaction of phosphorus with copperII ions is shown. Ignites or explodes with arsine phosphine silane diborane stibine red phosphorus white phosphorus boron active carbon silicon arsenic.
Phosphorus was discovered in 1669 and exists in four or more allotropic forms including white or yellow red and black or violet. Hazardous Substances Data Bank HSDB Hydroxyl radical reaction rate constant 4X10-14 cu cmmolec-sec at 25 C. Our specialists are available to advise you about the best products.
Group 15 elements include Nitrogen N Phosphorus P Arsenic As Antimony Sb and Bismuth Bi. 1542-50 2002 Hazardous Substances Data. Hawleys Condensed Chemical Dictionary 15th Edition.
Ignites sulfides at ambient temperature. CH 4 methane. The p-Block Elements Notes Class 12 Chemistry Chapter 7.
It isnt a good choice as a way of making chloroalkanes although it is used as a test. The temperature at which the liquidgas phase change occurs. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
NH 3 ammonia. Keep white phosphorus. Ignites as a liquid synthetic and natural rubber.
The p-block elements are placed in groups 13 to 18 of the periodic table. Reaction with phosphorusV chloride PCl 5. Hydrogen chloride HCl a compound of the elements hydrogen and chlorine a gas at room temperature and pressure.
Phosphorus trichloride CAS 7719-12-2 Methyl bromide CAS 74-83-9 Ethyl phosphonothioic dichloride CAS 993-43-1 Sulfur dioxide CAS 7446-09-5 Methyl chloroformate CAS 79-22-1 Ethyl phosphonic dichloride CAS 1066-50-8 Sulfuric acid CAS 7664-93-9 Methyl chlorosilane CAS 993-00-0 Ethyleneimine CAS 151-56-4 Tungsten hexafluoride CAS 7783-82-6 Methyl hydrazine. Sublimation The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. D Phosphorus reacts with chlorine to form phosphorus trichloride PCl 3.
Calculate the volume of O produced at 20ÂC. Solid phosphorusV chloride phosphorus pentachloride reacts violently with alcohols at room temperature producing clouds of hydrogen chloride gas. In which the valence shell of boron has only three pairs of electrons.
100 atm when 200g of 100 by mass H0 in water is treated with 100. Very reactive to nucleophiles hydrolyzed rapidly by water at room temperature. New York NY 2007 p.
The reaction is rapid at temperatures above 250 C 482 FThe reaction represented by the equation H 2 Cl 2. This is approximately the sum of the. It is more common has cubic crystal structure and at 1952 K 780 C it transforms into β-form which has.
CO 2 carbon dioxide. Density g cm 3 Density is the mass of a substance that would fill 1 cm 3 at room temperature. Hydrogen chloride may be formed by the direct combination of chlorine Cl 2 gas and hydrogen H 2 gas.
H 2 hydrogen. Relative atomic mass The mass of. Readily forms an explosive N-chloro derivative with aziridine.
The crystal structures were characterized by X-ray diffraction XRD using an X-ray diffraction system Rigaku Smart Lab Intelligent X-ray Japan with filtered Cu Kα radiation λ 15418 Å at 40 kV and 100 mA at room temperature in the 2θ range of 565 at a scanning speed of 15min. The temperature at which the solidliquid phase change occurs. White phosphorus is a colorless white or yellow waxy solid with a garlic-like odor.
H 2 0 2 decomposes slowly at room temperature to yield 0 2. α alpha and β beta. Some nonmetals are solids at room temperature while others are gases.
A solution of the gas in water is called hydrochloric acid. Alcohols react with liquid phosphorusIII chloride also called phosphorus trichloride to make chloroalkanes. O 3 ozone.
John Wiley Sons Inc. It is obtained by the combustion of phosphorus in a limited supply of air at low temperature. Their valence shell electronic configuration is ns 2 np 6 to ns 2 np 6 except He which has 1s 2 electronic configuration.
Electron-deficient molecules typically react with species. Phosphorus is poisonous with a fatal dose of just 50mg. The Exception to the Rule.
P 4 3 O 2 P 4 O 6. Phosphorus trioxide reacts with water to form phosphorous acid reflecting the fact that it. Martin P et al.
4 tetrahedron is also present in liquid and gaseous phosphorus up to the temperature of 800 C 1470 F when it starts decomposing to P 2 molecules. In general if a molecule. Similar arguments can be applied to boron trichloride BCl 3 which is a stable gas at room temperature.
H 2 O water. Boiling point The temperature at which the liquidgas phase change occurs. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry.
PCl 3 phosphorus trichloride. At room temperature the α-form is stable. Based on your experience is the vaporization a spontaneous process at room temperature.
White phosphorus exists in two crystalline forms. It does not occur naturally but is manufactured from phosphate rocksWhite phosphorus reacts rapidly with oxygen easily catching fire at temperatures 10 to 15 degrees above room temperatureWhite phosphorus is used by the military in various types of ammunition and to produce smoke for concealing troop. Density g cm 3 Density is the mass of a substance that would fill 1 cm 3 at room temperature.
CH 3 CH 2 OH ethanol. Phosphorus is insoluble in water and soluble in carbon disulfide and it burns spontaneously in air. Predict whether delta-S is positive or negative for this process.
