Branched Chains The following steps are taken in naming an alkane with a branched chain. Volatile fatty acids VFAs are linear short-chain aliphatic mono-carboxylate compounds such as acetic acid propionic acid and butyric acid which are the building blocks of different organic compounds.
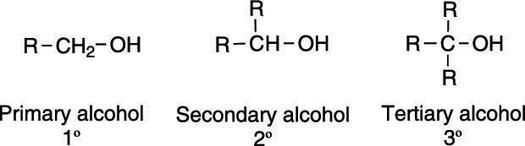
How To Classify And Name Alcohols Dummies
The longest chain of the hydrocarbon must be numbered and the locant numerical position of the substituent must be included to account for possible isomers.
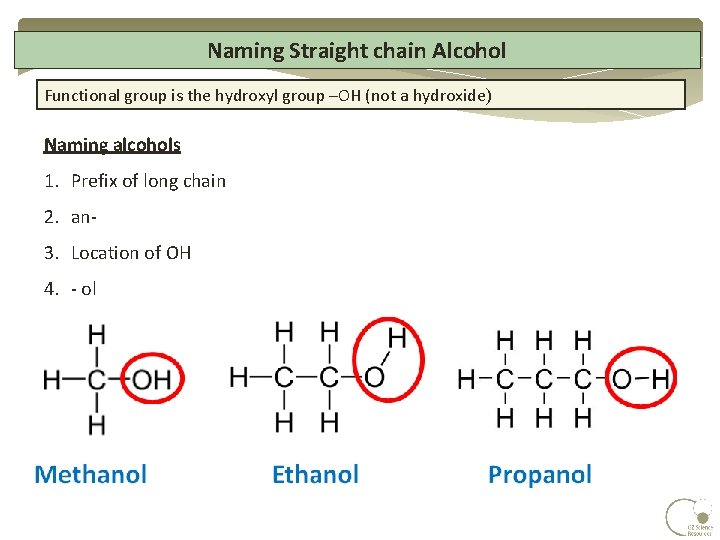
How to name branched chain alcohols. Even branched-chain alkanes are saturated because all the covalent bonds between carbon. The systematic names for branched hydrocarbons use the lowest possible number to indicate the position of the branch along the longest straight carbon chain in the structure. Structures II IV and V have a branched chain.
The carbon skeleton of organic molecules can be straight branched or ring shaped cyclic. A molecular formula can be written or a structural formula drawn from the systematic name of a straight-chain or branched alcohol that contains no more than eight carbon atoms in its. Fatty alcohols or long-chain alcohols are usually high-molecular-weight straight-chain primary alcohols but can also range from as few as 46 carbons to as many as 2226 derived from natural fats and oilsThe precise chain length varies with the source.
A Find the longest continuous carbon chain and select the appropriate alkane name from Table 1. A locator number is placed. If you are asked to write a IUPAC name for a compound pay careful attention to the syntax of this nomenclature system.
Other examples of IUPAC nomenclature are shown below together with the common names often used. Discovered by Chevrenl in 1913 cetyl alcohol is one of the oldest. If there are multiple side chains of the same type use prefixes such as di- and tri- to indicate it as such and number each one.
Now that the halogen has been identified the length of the carbon chain itself can be determined. How to name organic compounds using the IUPAC rules. In general the base part of the name reflects the number of carbons in what you have assigned to be the parent chain.
Most are typically very long which allows for a huge number. Note that a locator number is not needed on a two-carbon chain. As with double and.
IR of Alcohols and AminesIR of Alcohols and Amines. As a result of carbons unique combination of size and bonding properties carbon atoms can bind together in large numbers thus producing a chain or carbon skeleton. Some commercially important fatty alcohols are lauryl stearyl and oleyl alcoholsThey are colourless oily liquids for smaller.
They also can be exploited as a raw. CH 3 2 CCHCHOHCH 3 is 4-methyl-3-penten-2-ol. VFAs have two acetic acid to six caproic acid carbon atoms.
The general chemical formula of an alkane is CnH2n2 but since the halogen effectively replaces one of the iodobutane hydrogens the modified alkane formula is CnH2n1X where X is a halogen. Constitutional Isomers isomers that differ by Constitutional Isomers atomic connectivity Isomers that differ in how their atoms are arranged in atomic connectivity chains are called constitutional. The longest chain is named as an alkane and the final -e is changed to an -ol.
Straight-chain and branched alcohols can be systematically named indicating the position of the hydroxyl group from structural formulae containing no more than eight carbon atoms in their longest chain. They are extremely useful in the chemical industry due to their functional groups. Side chains are not included in the carbon count b Name all of the side.
When the carbon atoms all join up to form a ring we refer to these as cyclic compounds. Combine the elements of the name into a single word in the following order. However there is likely one more step.
We name these cyclic alkanes by placing the prefix cyclo in front of the name of the alkane. Nomenclature of Carboxylic Acids. For more complex alcohols the IUPAC nomenclature must be used.
Detour Whey Protein Bar contains 15g of protein rich in branched chain amino acids low in sugar and provides serious nutrition to athletes. The suffix of the name reflects the types of functional groups present on or. Solution i CH3 CH2.
It can be produced from the reduction of palmitic acidCetyl alcohol is present in a waxy white powder or flake form at room temperature and is insoluble in water and soluble in alcohols and oils. Also write their IUPAC names. The name of this compound is 2-ethylbut-13-diene.
Straight-chain and branched alcohols are named by first selecting the longest continuous chain of carbon atoms containing the carbon to which the OH group is bonded. Cs are straight-chain or normal alkanes Alkanes with one or more Cs connected to 3 or 4 Cs are branched-chain alkanes butane isobutane. These names are listed within the discussion of naming alkanes.
On longer chains the location of the hydroxyl group determines chain numbering. In order to name organic compounds you must first memorize a few basic names. Spelling must also be absolutely.
Look to the largest peak on your mass spectrum. For example commas and dashes must be used in precisely the correct manner and extra spaces must be avoided. Thus the systematic name for isobutane is 2-methylpropane which indicates that a methyl group a branch consisting of CH 3 is attached to the second carbon of a propane molecule.
Nomenclature of Aldehydes. Problem 131 Write structures of different chain isomers of alkanes corresponding to the molecular formula C6H14. Name each side chain by changing the suffix of the name of the alkane from -ane to -yl Number the longest continuous chain in order to give the lowest possible numbers for the side-chains.
Organic molecules are built on chains of carbon atoms of varying lengths. Name ending according to the functional group and its position along the longest carbon chain. Code no of carbons meth 1 eth 2 prop 3 but 4 pent 5 hex 6 hept 7 oct 8 non 9 dec 10 General rules for naming carbon chains Count the longest carbon chain and name appropriately Find any branched chains and count how many carbons they contain Add the appropriate prefix for each branch chain Eg -CH3 methyl or -C2H5.
Number and name the side chains before the name of the root chain. Thus you have seen that C4H10 and C5H12 have two and three chain isomers respectively. Cetyl alcohol also known as 1-hexadecanol or n-hexadecyl alcohol is a 16-C fatty alcohol with the chemical formula CH3CH215OH.
When naming branched chain alcohols be careful to number the longest possible carbon chain first. To name a branched hydrocarbon the name of the substituent is combined with the parent name of the hydrocarbon without spaces. Such structural isomers which differ in chain of carbon atoms are known as chain isomers.
When writing the name you follow the convention of using commas between numbers and dashes. Alcohols are usually named by the first procedure and are designated by an ol suffix. Carboxylic acids aldehydesketonesalcoholsalkeneshalogenoalkanes.
Straight chain compounds have higher boiling point then branched chain because in straight chain molecules are strongly entangled with each other like noodlesand have more contact with other molecules so strong force is required to remove such molecules consequently straight chain compounds have higher boiling point than branched compounds. Examples of Saturated Cyclic Hydrocarbon Compounds only single bonds between carbon atoms Molecular.