3Fe3 - Fe2 is now 3Fe3 - 3Fe2 6. The present invention relates to a drilling mud composition that contains aloe vera particles as a rheological modifier andor a filtration control agent and a process for.
The immobilization reaction was carried out at 4 C for 24 h under.

Formation reaction for potassium sulfite. This binding action actually serves to protect the wine. It is also a component in many drugs which helps maintain their potency and stability. A mixture of potassium chlorate and sodium amide explodes Mellor 8258.
On the other hand the reaction is an. Attempted isolation of the common salts of bisulfite results in dehydration of the anion with formation of metabisulfite S 2 O 2 5 also known as disulfite. The reaction was ended after 2 h and the sample was washed with 01 M sodium acetate buffer pH 68 to remove any residual chemicals.
Sodium Sulfite is a white crystal or powder with reducing property. The primary interaction with the bromide crystals is by Compton and photoelectric interactions thereby knocking out electrons. Potassium chlorate and sulfuric acid react to cause fire and possible explosions Mellor 2315.
As long as there is free sulfur present it is available to react with and effectively neutralize both oxidation and microbial spoilage threats. The outlet port chamber contains essential and nonessential amino acids with electrolytes. Commercially it is obtained as a by-product.
Because of this equilibrium anhydrous sodium and potassium salts of bisulfite cannot be obtained. Indications CLINIMIX amino acids in dextrose Injections and CLINIMIX E amino acids with electrolytes in dextrose with calcium Injections are indicated as a. CLINIMIX E sulfite-free amino acids with electrolytes in dextrose with calcium injection for intravenous use consists of sterile nonpyrogenic hypertonic solutions in a dual chamber container.
The optimal pH value for maximum effectiveness of sulphurous acid as a preservative would be 51 because it would then be present in its undissociated form. FDA has ruled. Sodium thiosulfate drug product may contain trace impurities of sodium sulfite.
Acidify about 2 mL of the test solution with 3 M H 2 SO 4 and then dissolve one-half spatula full of solid FeSO 47H 2 O in the acidified. To avoid confusion caused by differences in reaction conditions and ensure uniformity of data the scientific community has selected. But its good practice.
Binding continues until all of the various reaction-able elements in the wine have either become bound up or there is no more free sulfite to interact with. Sulphurous acid whilst present as an acid acts as a very strong preservative and the undissociated acid molecule is the active agent. For example a reaction between the dilute sulphuric acid and sodium sulphite will result in the formation of SO 2.
The latent invisible image formation is the ionization of the exposed silver bromide crystals by photon energy that emerges from the patient occurring in the emulsion layer before processing occurs. 2 HSO 3 S 2 O 2 5 H 2 O. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure.
The formulas for the individual electrolytes and amino acids are provided in Table 8. Standard Enthalpies of Formation. Con A was immobilized to the cellulose scaffold by a covalent bond through the grafted glutaraldehyde in a reaction containing 12 mg of Con A in 25 mL 01 M sodium acetate buffer pH 68.
Sodium sulfite potassium permanganate and sulfuric acid KMnO4 H2SO4 Na2SO3 are very common and well-known chemicals all around the world. Na 2 SO 3 H 2 SO 4 Na 2 SO 4 H 2 O SO 2. In the last reaction the nitrogen oxide reacts with excess Fe 2 to give the brown complex ion FeNO 2.
Further Physical and Organic Chemistry. If a drop of a solution of sulfur dioxide in ether or alcohol is added to powdered potassium chlorate the mass explodes Mellor 2311. 3 ion are also known as sulfite lyes.
Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. They react to form potassium sulfate ManganeseII sulfate water as well as sodium sulfate. During the oxidation reaction sulfonyl groups and sulfite groups were successfully introduced to the surface of the PPS films.
The presence of a trace amount of sulfites in this product should not deter administration of the drug for treatment of emergency situations even if the patient is sulfite-sensitive. 266 describe the oxidation of alcohols using acidified potassium dichromateVI with reference to formation of aldehydes and carboxylic acids from primary alcohols formation of ketones from secondary alcohols and resistance to oxidation of Unit A2 1. A process for fracking a geological formation whereby the drilling mud composition is injected into the geological formation through a well bore at a pressure of at least 5000 psi to fracture the geological formation.
Multiply the other half reaction by the of electrons to make the of electrons equal in each half reaction. It was first isolated from potash the ashes of plants from which its name derives. Please see accompanying Package Inserts for full Prescribing Information.
However there are some reports of anhydrous. Add it all together We probably didnt need to go this far since we already know the of H2O molecules. This agent was used by food industry to help maintain the fresh appearance of food products.
Its also available in almost all chemistry laboratories. In a subsequent reaction negatively charged SO 3 ions are obtained SO 3 2 2 H as well. There have been no controlled clinical trials conducted to systematically assess the adverse events profile of.
Through the formation of a black precipitate upon reaction with ferrous sulfate How does SIM agar Sulfide indole motility medium detect the production of hydrogen sulfide gas. Ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states. Thus a physical change occurs when the radiograph is exposed.
In effect free SO2 can be viewed as. Well just multiply the whole half reaction by 3. The water contact angle of.
PH of sulfite-free Amino Acid Injection and sulfite-free Amino Acid Injection with Electrolytes in the outlet port chamber was adjusted with glacial acetic acid. Potassium is a chemical element with the symbol K from Neo-Latin kalium and atomic number 19. It is the formation of this brown complex that is used to identify NO 3-called the brown ring test.
In the laboratory sulphur dioxide is prepared by the reaction of metallic sulphite or a metallic bisulphite with dilute acid. Sodium sulfite exhibits bleaching de-sulfurizing and dechlorinating activities. Thus if K sp Mm An is the equilibrium constant for the reaction M m A n s mMaq nA aq where M m A n is the slightly soluble substance and M and A-are the ions produced in solution by the dissociation of M m A n then the Gibbs energy change is.
The magnitude of ΔH for a reaction depends on the physical states of the reactants and the products gas liquid solid or solution the pressure of any gases present and the temperature at which the reaction is carried out. 42 Enthalpy entropy and free energy.
HAZARDS IDENTIFICATION Emergency Overview. When introduced to water sodium metabisulfite liberates.

How Much Sodium Sulfite Photo Net Photography Forums
Most sulfites are insoluble in water.

Solubility of sulfite. Aging barrels should also be adequately maintained particularly when they are empty. M m A n s mM n aq nA m-aq. A slurry is a thick suspension of an insoluble precipitate in water.
Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution. Solubility Product Constants near 25 C. This compound is highly soluble in glycerol but not very soluble in ethanol.
Trade Names and Synonyms. SOLUBILITY PRODUCT CONSTANTS The solubility product constant K sp is a useful parameter for calculating the aqueous solubility of sparingly soluble compounds under various conditions. A white water-soluble solid it is used commercially as an antioxidant and preservative.
Sodium sulfite sodium sulphite is the inorganic compound with the chemical formula Na 2 SO 3. The molar mass of sulfate is about 96 gmol. For ionic compounds with limited solubility in water an equilibrium constant K sp can be defined from the ion concentration in water from the equation.
Most sulfates are soluble in water. Acetic acid is a weak acid consequently it is written in molecular form. K sp M n m A m- n.
Significant attention must be paid to winery hygiene and equipment must be cleaned regularly during the harvest. Mainly anhydrous sodium sulfite is used for the removal of water from organic layer. GROUP I ALKALI METALS GROUP II ALKALINE EARTH METALS TRANSITION METALS POST-TRANSITION METALS Ammonium NH 4 Lithium Li Sodium Na Potassium K Magnesium.
A pale yellow aqueous solution with a pungent sulfur-like odor. Ammonium sulfite NH42SO3 479 54 608 688 784 104 144 150 153 Ammonium tartrate NH42C4H4O6 45 55 63 705 765 869 Ammonium thiocyanate NH4SCN 120 144 170 208 234 235 346 Ammonium thiosulfate NH42S2O3 173 205 269 Ammonium vanadate NH4VO3 048 084 132 178 242 305 70 Aniline C6H7N 36 Antimony trifluoride SbF3 385 444 562 dec Antimony sulfide Sb2S3. A saturated solution of sodium sulfite in water is mildly basic with an approximate pH value of 9.
From alkali metals only lithium forms insoluble carbonate. This website uses cookies to help provide you with the best possible online experience. The table below gives calculated values of K.
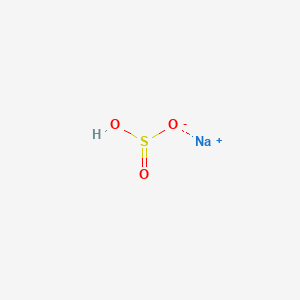
Sodium acid sulfite sodium hydrogen sulfite monosodium sulfite NaHSO3. FDA has ruled. CID 1100 Sulfurous acid CID 222 Ammonia Dates.
It is also a component in many drugs which helps maintain their potency and stability. Also called ammonia caramel. In aqueous solution it is only a few percent ionized.
It is corrosive to aluminum. Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solvent to form a solution of the solute in the solvent. Sulfite is an anion composed of sulfur and oxygen atoms.
Sodium sulfite is an inorganic salt with the chemical formula Na 2 SO 3. Sodium Sulfite is a white crystal or powder with reducing property. All salts of Group IA and ammonium are soluble.
Ag aq CH 3 COOHaq --- AgCH 3 COOs H aq Nitrate is the only spectator ion. Calcium sulfite or calcium sulphite is a chemical compound the calcium salt of sulfite with the formula CaSO 3 xH 2 O. All salts of nitrates chlorates and acetates are soluble.
Substance Substance name. Sodium sulfite exhibits bleaching de-sulfurizing and dechlorinating activities. 12 11062020 EN English US Page 1 SECTION 1.
Na 2 S 2 O 5 is fairly soluble in water its solubility corresponds to 653g100mL at a temperature of 20 o. So after separating the solvents residual water will remain the organic layer. With sulfite compounds sulfurous acid potassium sulfite potassium bisulfite sodium sulfite and sodium bisulfite.
All salts of carbonate phosphate and sulfite are. It may be determined by direct measure-ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states. The solution itself is relatively non-toxic and.
Thus if K sp Mm An is the equilibrium. Silver acetate is insoluble and you learn this from a solubility chart. Most often the solvent is a liquid which can be a pure substance or a mixture.
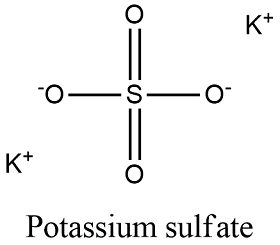
NaClaq Sodium chloride Na-aq Cl aq K 24 SO aq Potassium sulfate 2 K-aq SO 4 2aq Li 23 CO aq Lithium carbonate 2 Li-aq CO 3 2aq Na 34 PO aq Sodium phosphate 3 Na-aq PO 4 3aq NH 42 SO 4 aq Ammonium. The molar mass of sulfite is about 80 gmol. Sodium Sulfite Anhydrous Safety Data Sheet according to Federal Register Vol.
Such a solution can undergo crystallization to yield heptahydrate crystals of Na 2 SO 3. Determinations of the solubility of a salt may be made by reference to SOLUBILITIES OF IONIC COMPOUNDS. In its anhydrous.
Non-combustible but excessive heat may liberate sulfur dioxide gas that is strongly irritating to the eyes and mucous membranes. Modern stainless steel equipment is easier to clean than tanks made of concrete or wood. It is a strong irritant to skin and mucous membranes.
Sodium Sulfite Anhydrous CAS-No. In addition the development of these microorganisms is favored by low doses of sulfite and high pH. Ionic Compound Formula K sp.
A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air. No ammonium compounds are used. But when we study deeply about solubility of metal carbonates most of the carbonates are insoluble in water.
Ammonium bisulfite is colorless crystals which are soluble in water. Using the solubility guidelines provided in the lab manual for this experiment predict whether this stage of the scrubbing process will produce a slurry ie precipitate or a solution ie no precipitate of. One may also speak of solid solution but rarely of solution in a gas.
All forms are white solids. With ammonium reactant but no sulfite. It is an ionic compound containing two sodium cations Na and one sulfite anion SO 3 2-.
It is most notable as the product of flue-gas desulfurization. Water and dichloromethane is slightly soluble in each other. Anhydrous sodium sulfite is an insoluble.
Solubility of carbonates have a variation because there are soluble and insoluble carbonates. All salts of halides are soluble except those of silverI copperI leadII and mercuryI. It has a faintly pungent smell similar to SO 2.
Two crystalline forms are known the hemihydrate and the tetrahydrate respectively CaSO 3 ½H 2 O and CaSO 3 4H 2 O. All salts of sulfate are soluble except for barium sulfate leadII sulfate and strontium sulfate. Where M m A n is the slightly soluble substance and M n and A m-are the ions produced in solution by dissosiation of M m A n.
In the presence of ammonium compounds ammonium hydroxide ammonium carbonate ammonium hydrogen carbonate and ammonium. This agent was used by food industry to help maintain the fresh appearance of food products. Please read our Terms Conditions and Privacy Policy for information about.
Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3. Sodium sulfite has a white or whitish-yellow appearance in its solid-state. Soluble salts are written as their aqueous ions.
58 Monday March 26 2012 Rules and Regulations Issue date.
Nitrate is NO 3- so nitrite has the same charge but one less oxygen NO 2- 2. Rongalite is a chemical compound with the molecular formula Na HOCH 2 SO 2.

How To Write The Formula For Zinc Sulfide Zns Youtube
Tin Il oxide 44silver sulfite.

Zinc sulfite formula. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. Potassium nitrate KNO3 11. Tin II bicarbonate SnHCO32 10.
Sodium thiosulfate ____Na2S2O3_____ 3. Write the chemical formula for the following ionic compounds. SnSO42 tin IV sulfate 55.
Zinc iron II iron III gallium silver lead IV chloride ZnCl 2. 2 Write the name for each of the following compounds. Sulfur dioxide ____SO2_____ 2.
Lead IV dichromate PbCr2O72 7. 2 lead IV sulfite 2. Cu2S copper I sulfide 43.
C 12 H 22 O 11. NH4NO2 ammonium nitrite 51. 2- so sulfite has the same charge but one less oxygen SO 3 2- b.
Strontium acetate SrC2H3O22 3. This salt has many additional names including Rongalit sodium hydroxymethylsulfinate sodium formaldehyde sulfoxylate and Bruggolite. Both anions show resonance in their chemical structures.
Sodium carbonate Na2CO3 6. The oxidation state of oxygen in. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
Na Mg 2 Non. Both are anions bearing negative charges. The names are found by finding the intersection between the cations and anions.
Lead II chlorate PbClO32 2. Coating and surface treatment. 3 iron II sulfite 35 ZnNO 2 2 zinc nitrite 36 C 6H 12O 6 glucose 37 NiNO 3 2 nickel II nitrate 38 PCl 3 phosphorus trichloride 39 MnOH 7 manganese VII hydroxide 40 O 2 oxygen.
Fe 2 ferrous. One of the earliest methods of distinguishing between these ions used the suffixes -ous and -ic added to the Latin name of the element to represent the lower and higher oxidation states respectively. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3.
MnClO44 manganese IV perchlorate 44. The overall charge of the anion is -2 for both anions. C 12 H 22 O 11.
Sr 2 strontium. Alganic acid or Na-Alginate. Solubility Product Constants near 25 C.
Radium sulfite souj IOK2Cr207 12PbBr2 Level Il 13ZnS03 14NaHC03 15. Students enrolled in Dr. H 2 SO 4.
The compound and its derivatives. If you know that a sufate ion is SO 4 2-then to get the formula for hydrogen sulfate ion you add a hydrogen ion to the front of the formula. Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box.
Formula to name problems. Copper I sulfite Cu2SO3 8. Chemical Formula Writing Worksheet Two Write chemical formulas for the compounds in each box.
Zinc carbonate ZnCO 3 aluminum hypochlorite Aℓ C ℓO 3 calcium phosphate Ca 3PO 4 2 cadmium phosphate Cd 3PO 4 2 iron III sulfate Fe 2SO 4 3 mercury II chlorite HgC ℓO 2 2 potassium phosphite K 3PO 3 magnesium hydroxide MgOH 2 iron II chlorate FeC ℓO 3 2 cobalt II carbonate CoCO 3. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. ZnBr2 zinc bromide 45.
K2SO3 potassium sulfite 42. Chemical Formula 11 calcium bromate 12 carbon monoxide 13 potassium oxide 14 antimony tribromide 15 zinc phosphate. A 100-gram sample of a compound contains 365 grams of sodium 254 grams of sulfur and 381 grams of oxygen.
The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. Al2S3 aluminum sulfide 54. Na 2 SO 3.
Section B Write the formula of the ionic compounds containing polyatomic ions 1. BaCIO32 barium chlorate 53. Draganjacs Introduction to Chemistry CHEM1003 General Chemistry I CHEM1013 and General Chemistry II CHEM1023 classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names formulae and the charges for the common cations and anions listed below.
Ionic Compound Formula K sp. The transfer of electrons between metals and non-metals produces charged particles called ions. Both anions are composed of a sulfur atom and oxygen atoms bonded to the sulfur atom.
7 Li2SO3 lithium sulfite 8 Zn3P2 zinc phosphide 9 SrC2H3O22 trontium acetates 10 Cu2O copper I oxide 11 Ag3PO4 silver phosphate 12 YClO3 yttrium chlorate 13 SnS2 tin IV sulfide 14 TiCN4 titanium IV cyanide 15 KMnO4 potassium permanganate 16 Pb3N2 lead. Chemical formulas can be. 1 NaF is sodium fluoride 2 K2CO 3 is potassium carbonate 3 MgCl 2 is magnesium chloride 4 BeOH 2 is beryllium hydroxide 5 SrS is strontium sulfide 6 Cu 2S is copper I sulfide 7 ZnI 2 is zinc iodide 8 Ca 3PO 42 is calcium phosphate 9 NH 4I is ammonium iodide 10 MnNO 33 is manganese III nitrate.
Ammonium cyanide NH4CN 5. H hydrogen. Chemical Formula Nomenclature Practice.
Fe 3 ferric. Copper Il acetate 42. Zinc phosphate Zn3PO42 4.
The names are found by finding the intersection between the cations and anions. And enables it to take a high finish. When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions.
Zinc Sulfite ZnSO 3 Magnesium Sulfite MgSO 3 Potassium Sulfite K 2 SO 3 Similarities Between Sulfate and Sulfite. Sodium chloride NaCl and magnesium oxide MgO. MgF2 magnesium fluoride 52.
1304-40-1 BaSi 2 O 5. An ionic compound is composed of a metal and a non-metal. Ca 2 calcium.
Sn 2 stannous. Complete these in lab and on your own time for practice. Does the empirical formula for the compound lead you to believe it is sodium sulfite o.
Li3P04 17SnC12 19Rb2Cr04 20KMn04 21CuCl 22FeS04 39chromium Ill chloride 40. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another. It is water-soluble and generally sold as the dihydrate.
Microsoft Word - Ionic_CovalentNameRace-1doc Created Date. Sulfate ion formula. It is listed in the European Cosmetics Directive as sodium oxymethylene sulfoxylate.
Metals lose electrons to produce positve ions called cations. You should complete this by Sunday. Give the formula for the following.
Some metals form positive ions in more than one oxidation state. Chemical formula plays an important role in understanding different concepts of chemistry. K potassium.
C 4 H 4 O 2. Parentheses and a subscript are not used unless more than one of a polyatomic ion is present in the formula unit eg calcium sulfate CaSO 4 not CaSO 4. Na-C 6 H 8 O 6n.
H 2 SO 3. Cations Anions zinc iron II iron III gallium silver lead IV chloride. Cadmium phosphate Cd3PO42 9.
Lithium sulfite 28 Zn 3 P 2 zinc phosphide 29 SrC 2 H 3 O 2 2 strontium acetate 30 Cu 2 O copper I oxide 31 Ag 3 PO 4 silver phosphate 32 YClO 3 yttrium I chlorate 33 SnS 2 tin IV sulfide 34 TiCN 4 titanium IV cyanide 35 KMnO 4 potassium permanganate 36 Pb 3 N 2 lead II nitride 37 CoCO 3 cobalt II carbonate 38 CdSO 3. Since a hydrogen ion has a 1 charge the net charge on. It gives paper a greasy or soapy feel.
Use the stock form for the transition metals. Students will write the formula and determine if compound is a strong or weak electrolyte.
To make Precipitated CaCO 3 is used as Filler and in Coating. Regardless of its ill-defined nature sodium bisulfite.
Sodium Bisulfite Nahso3 Pubchem
Used in Sulfite pulping.
Sodium hydrogen sulfite. This agent was used by food industry to help maintain the fresh appearance of food products. An ionic compound is made up of one or more anions and. Sodium acid sulfite sodium hydrogen sulfite monosodium sulfite NaHSO3.
Sodium bisulfite appears as white crystals or crystalline powder. Under Food Regulations Notes 223 223 Sodium metabisulfite Sodium salt of. In Pulp Bleaching.
They are both effective antioxidants that slow browning reactions and enhance ascorbic acid retention in certain fruit products as well as inhibitors of selected microorganisms which makes them useful in the winemaking. Each vial contains 125 grams of sodium thiosulfate in 50 mL solution 250 mgmL. Alkaline Pulping Process Chemical Recovery Bleaching Lime Stone.
Thus formed H₂S gas which is colorless combines with H₂S indicators iron bismuth or lead present in the medium producing insoluble heavy metal sulfides that appear as a black precipitate. LiBr lithium bromide. Na 2 S 2 O 5 H 2 O 2 NaHSO 3.
This is the list of all compounds with density tables present in the full database - it was actual on May 4 th 2005 as the list is growing it may be already incomplete. Hydrogen sulfite or bisulfite HSO 3-hydrogen phosphate HPO 4 2-hydroxide OH-hypochlorite ClO-nitrate NO 3-nitrite NO 2-oxalate C 2 O 4 2-perchlorate ClO 4-permanganate MnO 4-peroxide O 2 2- phosphate PO 4 3-phosphite PO 3 3-silicate SiO 3 2-sulfate SO 4 2-sulfite SO 3 2-thiocyanate SCN-thiosulfate S 2 O 3 2-Advertisement Ionic Compounds. Wine Making Food and Beverage Ecig Ingradeients.
And hydrogen cyanide HCN forms hydrocyanic acid. Sodium Sulfite is a white crystal or powder with reducing property. The first number under the column headed RQ is the reportable quantity in pounds.
Pore size 02 μm pore size 05 μm cartridge nominal length 5 in. H 2 O 2. Hydrogen bromide HBr forms hydrobromic acid.
221 221 Sodium sulfite Sodium salt of sulphurous acid Regulation 192b 4th Schedule Part I 222 222 Sodium hydrogen sulfite Sodium salt of sulphurous acid Regulation 192b 4th Schedule Part I 6 INS Number E Number Food Additive Name Name of additive as listed in Food Regulations Regulation Schedule No. Fisher Scientific 1 Reagent Lane Fair Lawn NJ 07410 For information call. Trade Names and Synonyms.
Department of Chemistry University of Texas at Austin I. Sodium sulfite exhibits bleaching de-sulfurizing and dechlorinating activities. 125 cm Code 7 EPDM rubber seal.
Sodium bisulfite in fact is not a real compound but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. Sodium chloride Sodium carbonate Sodium hydroxide Sodium sulfate Sodium phosphate. AC223070000 AC223070010 AC419440000 AC419440010 AC419440025 AC419440050 AC419441000 S654-3 S654-3LC S654-500 Synonyms.
The number in parentheses is the metric equivalent in kilograms. Hydrogen sulfide is also produced by the reduction of thiosulfate in anaerobic respiration by the enzyme thiosulfate reductase. For example hydrogen chloride HCl dissolves in water to form hydrochloric acid.
Non-combustible but excessive heat may liberate sulfur dioxide gas that is strongly irritating to the eyes and mucous membranes. The main target users are workers and those responsible for occupational safety and health. Compounds the most common of which are hydrogen sulfide methyl mercaptan dimethyl sulfide and dimethyl disulfide all with extremely low odor thresholds.
Sodium bisulfite or sodium bisulphite sodium hydrogen sulfite is a chemical mixture with the approximate chemical formula NaHSO 3. Hydrogen Peroxide Luolgs Iodine and Water. Each mL also.
Sodium thiosulfate injection is a sterile aqueous solution and is intended for intravenous injection. FDA has ruled. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes.
The primary aim of the cards is to promote the safe use of chemicals in the workplace. The ICSC project is a common undertaking between the World Health Organization WHO and. It is also a component in many drugs which helps maintain their potency and stability.
Sodium Thiosulfate Injection is a cyanide antidote which contains one 50 mL glass vial containing a 25 solution of sodium thiosulfate injection. The hydrogen sulfide producing bacteria H 2 S comprise various groups of bacteria and archaea that obtain energy by reducing various compounds that possess sulfur in their molecule including organic compounds sulfur amino acids and inorganic compounds with oxidized sulfur such as sulfate sulfite thiosulfate tetrathionates or elemental sulfur to H 2 S. Hydrogen Li lithium Be2 beryllium Na sodium Mg2 magnesium K potassium Ca2 Rb rubidium Sr2 strontium Cs cesium Ba2 calcium barium Fr francium Ra2 radium B boron C carbon nitride N3-oxide O2-fluoride F-neon Ne Al3 aluminum Si silicon phosphide P3-sulfide S2-chloride Cl-argon Ar helium He Zn2 zinc In3 indium Ge4 germanium As3-arsenide selenide Se2-bromide Br-krypton Kr.
HAZARDS IDENTIFICATION Emergency Overview. Many of the oxygen-rich polyatomic negative ions in Table 21 form acids that are named by replacing the suffix -ate with -ic and the suffix -ite with -ous. Choose from an industry-leading range of more than 1100 enhanced-quality food grade materials which meet European Regulatory Requirements.
Check our FAQ section for more details. Hydrogen chloride Hydrogen carbonate. A select range of products carefully manufactured for the alternative therapy market includes.
Different values of the pure water 0 concentration density reflect the fact that the measurements were done in different temperatures. Hydrogen sulfide is a. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses.
The major source of hydrogen sulfide is the direct contact evaporator in which the sodium sulfide in the black liquor reacts with the carbon dioxide in the furnace exhaust. Sodium bisulfite Catalog Numbers. Name the following Ionic Compounds.
Hydrogensulfite is the ion HSO 3Salts containing the HSO 3 ion are also known as sulfite lyes. The bisulfite ion IUPAC-recommended nomenclature. With operations in 65 countries Hanna is working to solve some of the worlds biggest problems by making scientific testing more accessible easy and accurate.
Sulfites including sodium sulfite sodium metabisulfite and sulfur dioxide are used as preservatives in processed fruit products including dried fruits juices and wine. Strong irritant to skin and tissue. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
Sodium bisulfite is used interchangeably with sodium metabisulfite Na 2 S 2 O 5Sodium metabisulfite dissolves in water to give a solution of Na HSO 3. The solution itself is relatively non-toxic and. Acetic acid acetic aldehyde acetone.
Distilled Deionised and Purified. A pale yellow aqueous solution with a pungent sulfur-like odor. CID 1100 Sulfurous acid CID 5360545 Sodium Dates.
Sodium bicarbonate 21 mg 025 mmol and sodium sulfite 63 mg 05 mmol were dissolved in degassed water 10 ml and the pH of the resultant. It is a white solid with an odour of sulfur dioxide. Water Hydrogen sulfate Hydrogen phosphate Hydrogen nitrate RevisedST72913.