Halogen containing universal flame retardant additive to blend with existing waterborne binder systems. Under prolonged exposure to fire or heat the containers may rupture violently and rocket.
Antimicrobial Ink and Stain Resistant Performance Plus Custom or Bisphenol A BPA.
Halogenated organic flame retardants. Trifluoromethane is a colorless nonflammable gas. The carbon chain length of commercial chlorinated paraffins is usually between 10 and 30 carbon atoms. No Flame Retardants ex.
Not all flame retardants present concerns but the following types often do. Melamine-based flame retardants show excellent flame retardant. 24 2021 PRNewswire -- The global flame retardant market size is anticipated to reach USD 1299 billion by 2028 registering a.
Halogenated Flame Retardants. Kannan December 2006 Volume 65 Issue 11. Transparent 0 Semi-transparent 0 Opaque 1 Flammability.
Reagecon worlds largest producer of Physical Chemical Standards supplier of Laboratory Equipment Consumables and Chemicals. Halogen free waterborne concentrate of phosphorus flame retardant which is virtually insoluble in water and organic solvents. Melamine derivatives ie salts with organic or inorganic acids such as boric acid cyanuric acid phosphoric acid or pyropoly-phosphoric acid and.
The organic PFRs are discussed in the following paragraphs Leisewitz et al 2000 Schmitt 2007. From waste prevention re-use and recycling to recovery and disposal. Resorcinol-bisdiphenylphosphate RDP is an aryl phosphate which is applied as an additive FR.
It is shipped as a liquid under pressure. Short-chained chlorinated paraffins is. SCCPs can be used as a plasticizer in rubber paints adhesives flame retardants for plastics as well as an extreme pressure lubricant in metal working fluids.
Chlorinated paraffins are produced by chlorination of straight-chained paraffin fractions. Ideally suited to application on. Many organic compounds are closely related to the alkanes.
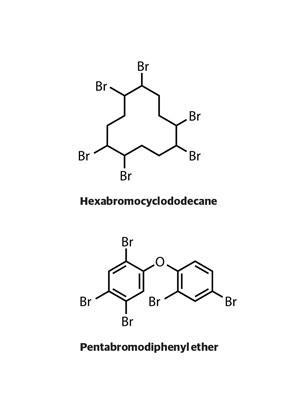
They are structurally akin to polychlorinated diphenyl ethers. Halogenated Persistent Organic Pollutants Dioxin 2004. Presence of halogenated flame retardants represents a major issue in the recycling of plastics of electronic displays.
As we noted in Section 17 Chemical Properties of Alkanes alkanes react with halogens to produce halogenated hydrocarbons the simplest of which have a single halogen atom substituted for a hydrogen atom of the alkane. Halogenated alkanes in land. Polybrominated diphenyl ethers or PBDEs are a class of organobromine compounds that are used as flame retardantsLike other brominated flame retardants PBDEs have been used in a wide array of products including building materials electronics furnishings motor vehicles airplanes plastics polyurethane foams and textiles.
Even more closely related are the cycloalkanes compounds in. Our Standard C-Zero Plus and Tailored C-Zero Plus fabrics are GREENGUARD Gold Certified meaning they meet stringent chemical emissions requirements and have been screened for various volatile organic compounds and along with the foam laminated to the backside of the fabric are free from halogenated brominated and chlorinated flame retardants while still meeting the federally-required. Melamine homologues such as melam melem and melon.
It may be narcotic in high concentrations. No POPs Persistent Organic Pollutants. It can be blended in existing waterborne binder systems and used as abase for intumescent coatings.
Small 7 Medium 16 Large 30 Opacity. ASTM E 84 Adhered Class A 128 ASTM E 84 Adhered Class B 0 ASTM E 84 Unadhered Class A 2 BS 5852 Crib 5 0 CA TB 117 E 0 CA TB 117-2013 2 0 0 0 0 0 H32 1 0 0 0 0 IMO FTPC 5 11 IMO. 742 Halogenated Flame Retardants 7421 Brominated Flame Retardants 7422 Chlorinated Flame Retardants 743 Halogen-Free Flame Retardants 7431 Phosphorus 7432 Aluminum Trihydroxide.
Brominated Flame Retardants BFRs in the Environment - Papers presented at the Third International Workshop on Brominated Flame. SAN FRANCISCO Nov. Organic 9 Plaid 1 Solid.
1 halogenated flame retardants also known as organo-halogen flame. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates indoles terpenes acetogenins and phenols. No PVC Polyvinyl Chloride.
Phosphorus flame retardants 2311. Transparent 1 Semi-transparent 0 Opaque 0 Flammability. More than 1600 halogenated organics have been identified with bromoalkanes being the most common haloalkanes.
They are used as flame retardants fire extinguishants refrigerants. No PBDEs Polybrominated Diphenyl Ethers. Alaee June 2006 Volume 64 Issue 2.
Over 99 of solvents recaptured and recycled. It is used as a substitute for halogenated FRs as well as for TPhP because it has a lower volatility a higher thermal stability and a higher P-content in. No Plasticizers Heavy Metals Stabilizers Phthalates.
Rotard April 2007 Volume 67 Issue 9. When heated these retardants degrade into toxic substances in gaseous form which then mix with. Phosphorus-based flame retardants both chlorinated and non-halogenated are extensively used in flexible and rigid polyurethane foams and the demand is anticipated to augment in the coming yearsThey have wide application prospects and it is a vital part of inorganic flame retardants.
Waste management is responsible for the entire waste cycle. Some halogenated compounds have been restricted by Directive 201165EU of the European Parliament and of the Council 13 because of their high toxicity but may be still found in old displays and others are still allowed. Flame retardants are chemicals incorporated in construction materials during manufacturing to slow down or stop the spread of flames either by forming a protective film or by inhibiting chemical reactions that support combustion in case of a fire break out.
Organic solvents are known as carbon-based solvents and their general property is primarily based on their volatility boiling point the molecular weight and color. Organic 0 Plaid 0 Solid. ASTM E 84 Adhered Class A 1 ASTM E 84 Adhered Class B 0 ASTM E 84 Unadhered Class A 0 BS 5852 Crib 5 0 CA TB 117 E 0 CA TB 117-2013 0 0 0 0 0 0 0 0 0 0 0 0 IMO FTPC 7 0 0 0 0 0 NFPA 701 TM1 0 0 0.
Speight in Environmental Organic Chemistry for Engineers 2017 7 Flame Retardants. Flame retardant chemicals are used in commercial and consumer products such as furniture and building insulation to meet flammability standards. In this family of non-halogenated flame retardants three chemical groups can be distinguished.
The suffix of the name reflects the type s of functional group s. It contains examples with substituents cycloalkenes.

Saat On Twitter Important Revision Of Organic Compounds Standardtest تحصيلي تحصيلي كيمياء S In 2021 Organic Chemistry Teaching Chemistry Organic Chemistry Study
1 1 2-Epoxy propane 2 Propylene oxide 3 1 2Oxo propane 4 1 2Propoxide.

Organic chemistry iupac naming practice. When naming organic compounds there are strict rules regarding punctuation. IUPAC name is 22- dimethyl propane. This video is a MUST for breaking down.
Organic chemistry naming examples 2. The examples cover the nomenclature of alkanes bicyclic compounds alkenes alkynes alcohols alkyl halides aromatic compounds aldehydes and ketones amines ethers and carboxylic acid derivatives such as nitriles esters and. The first video shows you how to break down the name of an organic molecule using my puzzle piece approach.
Play this game to review Organic Chemistry. The IUPAC name of the compound is. IUPAC Nomenclature Practice Problems - Chemistry Steps.
It provides an unambiguous structure. Use numbers commas dashes and spaces in your names wherever appropriate. Complete your research with top quality products.
Preview this quiz on Quizizz. What is the organic. This is a set of practice problems on naming organic compounds.
A hyphen is used to sep. State the correct IUPAC name of the following hydrocarbons. For naming purposes the functional groups are assigned with priorities Table 23.
A comma is used to separate two numbers. The syntax required is very specific. Solved Jun 16 2020.
Ad Enabling you to solve the toughest problems in life science. A systematic method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry IUPAC. These names are listed within the discussion of naming alkanes.
A simplified version Introductory IUPAC Organic Nomenclature is also available for high school andor college or. 80 thoughts on Organic Chemistry IUPAC nomenclature Practice Sheet with answers. Hence option 4 is the answer.
In general the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. Version 30 Updated 2014. If the compound includes more than one functional groups the one with the highest priority is the parent structure and determines the parent name.
The other groups will be regarded as substituents. Official IUPAC naming recommendations are not always followed in practice and the common or trivial name may be used. In order to name organic compounds you must first memorize a few basic names.
Some of the worksheets displayed are naming organic compounds practice practice 8 1 give the iupac name of each of the following naming organic compounds bcpldtpbc note 201306 acc j naming hydrocarbons work and key nomenclature in organic chemistry chemistry 1a nomenclature. Preferred names in the nomenclature of organic compounds. Nomenclature of Inorganic Chemistry.
Suffix is used to indicate the name of the parent structure and prefix is for the substituent. Naming alkanes cycloalkanes benzene derivatives. To review hydrocarbon nomenclature and see some examples click the button below.
Organic chemistry naming examples 4. Extension and Revision of the Nomenclature for Spiro Compounds Including Bicyclic Compounds IUPAC Recommendations 1999. These materials provide a step-by-step guide to learning organic nomenclature and are intended for those taking Introductory Organic Chemistry at a college or university.
The following guidelines for organic nomenclature are based on the def. Draft 7 October 2004. Complete your research with top quality products.
For naming organic compounds - some have had limit scope or become embedded in common us age and some have persisted over time The International Union of Pure and Applied Chemistry IUPAC periodically reviews naming practice attempting to standardise nomenclature. Name the unsaturated compounds. Hence option 1 is the answer.
Assign A Systematic Iupac Name To Each Of The Following Aldehydes And Ketones Practice Problems Ketones Chemistry Organic Chemistry. What is IUPAC nomenclature. Organic Chemistry Reactions 10th Higher Ed Worksheet Chemistry Worksheets Organic Chemistry Reactions Organic Chemistry.
The rules for naming organic compounds are tedious and can become overwhelming fast. Provide the IUPAC name for this 2-methylbutyl-substituted alkane. March 1 2014 at 100 pm.
Naming two isobutyl groups systematically. Video 1 Introduction To IUPAC. Organic chemistry naming examples 3.
Ad Enabling you to solve the toughest problems in life science. Your sample paper for nomenclature practice sheet for class xi cbse shall i have your solution this respect. Naming Organic Compounds Worksheet.
The IUPAC name of CH 3 COCHCH 3 2 is 1 2-methyl-3-butanone 2 4-methylisopropyl ketone 3 3-methyl-2-butanone. This organic chemistry video tutorial explains how to name alkenes using the iupac nomenclature system.
C bonded to N C N Thiol. These flashcards will equip you with content you need to succeed.

Table Of Functional Group Priorities For Nomenclature Master Organic Chemistry Organic Chemistry Organic Chemistry Study Chemistry Education
Organic Functional Group List Functional Group Compound PrefixSuffix Example IUPAC Name Common Name R-H alkane -ane CH 3CH 3 ethane CC alkene -ene H 2CCH 2 ethene ethylene CC alkyne -yne HCCH ethyne acetylene R-X haloalkane halo- CH 3Cl chloromethane R-OH alcohol -ol hydroxy- CH 3OH methanol R-NH 2 amine -amine amino- CH 3CH 2NH 2 ethylamine.

Functional groups in organic chemistry chart. Organic chemists can tell a lot about a molecule by the. MCAT Organic Chemistry Kaplan Guide. In addition to the chart three keys are available that organize the reactions according to mechanism functional group preparations and functi onal group reactions.
In that case you will have to determine which functional group has the highest priority in naming by using a functional groups naming chart. Ad Best-in-class lab materials technologies services to help you complete your research. These groups of atoms contain oxygen or nitrogen or sometimes sulfur attached to a hydrocarbon skeleton.
C bonded O of a hydroxyl group C OH Ether. Right-click the image below then select save as or print for the full version. The Chemistry of Garlic.
Functional groups in organic compounds. The carbonyl group is a super function because many common functional groups are based on a carbonyl including. Ad Best-in-class lab materials technologies services to help you complete your research.
Infrared is a powerful identification tool for functional groups because of the similar absorption frequencies for those groups in different molecules. In particular sulfur-containing functional groups are much better represented and a few additional nitrogen-containing groups have crept in. Expertise on every level to craft science technology solutions in life science.
Since I published my original functional groups chart back in 2014 Ive had a fair few requests to expand it to include more functional groups. Functional groups are an atom or a group of atoms bonded together in a unique manner attached to the alkyl chain or ring or any other organic molecule. The purpose of this chart will be clear if youve got a background in chemistry.
In Organic Chemistry you will come across molecules that contain more than one type of functional groups. The actual frequency is affected by the environment so the reference chart shows wide bands rather than specific frequencies. 6 Sets - 1099.
Dont forget about conjugated alkenes too as they are important in many organic processes such as the Diels-Alder reaction. Following is the table of the common functional groups you will encounter in organic chemistry. The knowledge of some basic functional groups and how they react would give us tremendous leverage to tackle the problem of predicting chemical reactivity in organic chemistry.
Tags science functional groups organic chemistry orgchem orgo. Functional groups are structural units within organic compounds defined by specific atom and bond arrangements. While alkanes and cycloalkanes are not particularly reactive alkenes and alkynes definitely are.
C bonded to halogen CC bonded to halogen C-X Alcohol. Organic chemistry functional groups chart Nomenclature priority and properties. Chemistry can be thought of a search for order in matter and this chart attempts to provide some insight into the order that.
The Functional Groups R-Z Functional Group Z Name Suffix or Prefix Used in Systematic Name-OH-OR-R Alkane Alkene Alkyne Arene-X Cl Br I or F Haloalkane Alcohol Ether-NH 2 Aldehyde Ketone Carboxylic Acid Ester Amide Amine-ane-ene-yne not responsible halo--ol not responsible-al-one-oic acid-oate-amide amino-Nomenclature Review methan- ethan- propan-. The structure of capsaicin the compound responsible for the heat in peppers incorporates several functional groups labeled in the figure below and explained throughout this section. This group is alkanes cycloalkanes alkenes and alkynes.
Expertise on every level to craft science technology solutions in life science. You will not be. The carbonyl group gjr-- Carbonyl group CO analogous to alkene Two groups aldehydes RCHO and ketones R2CO Aldehyde 18 H H O methanal formaldehyde H3C H O ethanal acetaldehyde H O benzaldehyde HO O OH OH OH OH H 2R3S4R5R-23456-pentahydroxyhexanal glucose Ketones H3C CH3 O propanone acetone CH3 H O H3C R-2-methyl-5-prop-1-en-.
Functional groups are collections of atoms in organic chemistry molecules that contribute to the chemical characteristics of the molecule and participate in predictable reactions. Functional groups are structural units within organic compounds that are defined by specific bonding arrangements between specific atoms. In simple terms a functional group with MORE bonds to oxygen will outrank one with fewer bonds.
Carboxylic acid 3 ketone 2 alcohol 1 bromine 0. Functional Groups Name Structure alkane C C alkene C C alkyne C C diene arene alkyl halide C C X X F Cl Br I X aryl halide X F Cl Br I alcohol C C OH OH phenol ether C O C O epoxide O ketone C C C O aldehyde C C H O carboxylic acid C C OH Ending -ane -ene -yne -diene none none none -ol. C bonded to SH group C SH.
Functional Groups Alkyl Halides Alcohols and Ethers Aldehydes and Ketones The Carbonyl Group Amines Alkaloids and Amides Grignard Reagents. Functional Groups with Carbon Singly Bondd l ided to an Electronegative Atom Alkyl halideAlkyl halide. Esters have a pair of alkyl or aromatic groups attached to a.
4 19 Organic Chemistry on the MCAT is full of functional groups and famous reactions. Aldehydes ketones carboxylic acids esters amides acyl acid chlorides acid anhydrides. List of functional groups in organic chemistry.
This week I finally got around to doing that. They confer special characteristics and properties to the molecule they are attached. Two Cs bonded to the same O C O C Amine.
Research in the 1990s. These keys are included on this site. If you havent its a useful tool to decode the different parts that make up molecules in organic chemistry.
This is one of many videos provided by Clutch Prep to prepare you to succeed in your college classes. Skeleton structures are like a short representation of an organic molecule to avoid the complicated structure of large and big molecules or compounds that are branched.

Organic Chemistry Wallpaper Google Search Organic Chemistry Organic Chemistry Study Chemistry Basics
The convention is makes it easier to draw molecules but the convention does need to be learned.

Organic chemistry skeletal structure. The skeletal system consists of 206 bones in the adult body. The carbon-hydrogen bonds are not shown but can be calculated by subtracting the. For instance if we wanna draw a line representation of 2-butanol well get the following picture.
All organic molecules have a hydrocarbon chain. A representation of molecular structurein which covalent bondsare shown as lines. They make up the words of every organic chemistry sentence so it is vital to understand how to read and write structures.
This organic chemistry video tutorial shows you how to draw lewis structures bond line structures and skeletal structures including condensed structural for. To apply and interpret the short-line structures correctly it is very. Skeletal structures are an effecient way to draw these molecules by using lines to represent C-C bonds at each vertex or line-end.
In the skeletal structures we show the absolute minimum that we need to represent a molecule in a non-ambiguous way. In this article I will help you understand the nature of the skeletal formula and how it applies to organic molecules. We have detected that you are are on a small device such as a mobile phone.
In organic chemistry a skeletal formula shows just the carbon skeleton or carbon backbone and functional groups. Please click here to perform a new search. Skeletal Structural Formula Skeletal Structure or Skeletal Formula Chemistry Tutorial Key Concepts.
Thus skeletal drawings have certain rules we use to draw them. Double bonds are shown as a double line and hetero atoms are draw as usual. Connective tissue and collagen forms bones.
In a skeletal formula each carbon is represented by an angle or termination in a line and the hydrogen atoms are just assumed. Enter single file name. Skeletal structures is the most common type of the molecular representation in organic chemistry.
Saved structure search file. Video explaining Skeletal Structure for Organic Chemistry. More commonly organic and biological chemists use an abbreviated drawing convention called line-bond structures also sometimes called skeletal structures.
Drawing Skeletal Structures Practice Questions for Organic Compounds- Test your understanding and skills on how to draw organic chemistry molecules in simple line structure or skeletal structure. Carbons and Hydrogens are implicit. Be ready for your next Orgo exam.
The carbon-hydrogen bonds are not shown but can be calculated by subtracting the. The structure drawing can be further simplified by short-line structure or bond-line structure skeletal formula in other books with most atoms omitted it is also the very common type of structure formula used in Organic Chemistry because of its simplicity. Practice quiz associated tutorial video.
Skeletal formula skeletal structure. Skeletal structure because of minerals such as calcium and phosphate deposits in their. With threshold match stereochemistry and Molecular Formula with Sort results by.
All organic molecules have a hydrocarbon chain. We recommend you use a larger device to draw your structure. A certain amount of logical reasoning must be used to understand.
The symbols for all elements other than carbon and hydrogen are always drawn unless part of a groupabbreviation such as Ph. This is another way to represent the molecular structure. Skeletal structures are an effecient way to draw these molecules by using lines to represent C-C bonds at each vertex or line-end.
A skeletal structure may also be referred to as a skeletal structural formula. A skeletal formula does NOT explicitly show. Use our editor to draw your structure.
Organic Chemistry Molecular Representations Dos and Donts of the Skeletal Structure Drawings When drawing the skeletal structures remember that each dot connecting the lines is a carbon atom. Understanding skeletal formula in organic chemistry will be the key to your success when it comes to working through organic chemistry reactions and step by step mechanisms. Chemical structures are usually represented by the skeletal formula which provides a graphical representation of the molecule with most hydrogens omitted for clarity.
Bones consist of arteries veins axon nerve fiber and cartilage.
