Its structure is given below Image will be uploaded soon. 11545 K anhydrous 122.
Answered What Is The Molarity M Of A N 10 Bartleby
Potassium carbonate K 2 CO 3 2.
Sodium hydroxide formula and molar mass. Substitute the known values to calculate the molarity. 1528 gcm 3 20 C anhydrous 145 gcm 3 20 C trihydrate Melting point. To learn about the structure Properties Preparation Uses Health Hazards and FAQs of Sodium hydroxide NaOH.
Sodium sulfate Na 2 SO 4. Make sure to. Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound.
Sodium hypochlorite pentahydrate is a greenish yellow solid. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Sodium hydroxide is also the most common base used in chemical laboratories.
You can convert between wv mv or weight ratio percentage concentration and molarity mol L-1 using the molar mass of the solute. CuCl 2 CuCl2 C 12 H 22 O 11 C12H22O11 C 6 H 5 3 PCCO C18H15PCCO Formula. Solubility Table Table of Solubilities Water is a commonly used solvent so it is very useful to construct a table of solubilities based on the mass of a solute that will dissolve in a given volume of water.
Vinegar acetic acid odor when heated to decomposition. An alkali caustic soda is widely used in many industries mostly as a strong chemical base in the manufacture of pulp and paper textiles drinking water and detergents. 597 K anhydrous 58 C 136 F.
Exhaust from a chimney contains 10 mol of oxygen O 2 53 mol of nitrogen N 2 and 37 mol of carbon dioxide CO 2. Convert the expressions above to obtain a molarity formula. Soda caustic CAS.
Properties of Sodium Hypochlorite. The molar concentration of sulphuric acid is 400 mol L 1. Sodium hydroxide MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents.
Calculate the mass of sodium sulfate made when 20 g of sodium hydroxide reacts with excess sulfuric acid. It is also commonly known as caustic soda or Iye and is widely used in manufacturing a variety of products such as paper soap and detergents pulp explosives liquid drain and oven cleaners etc. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH.
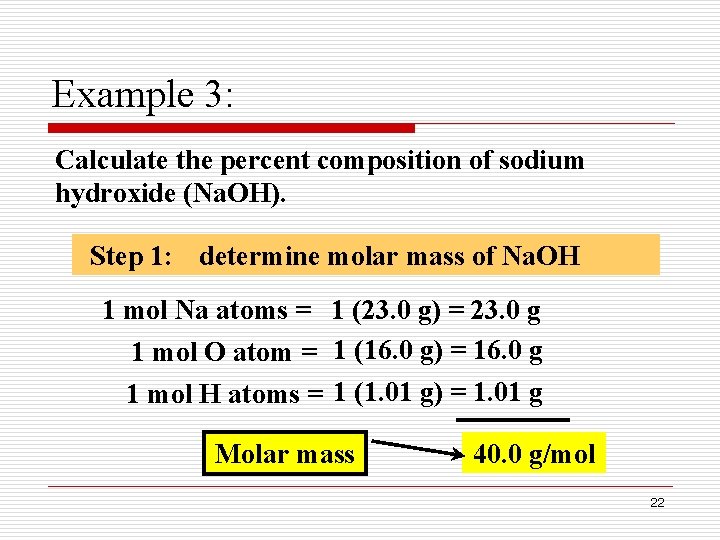
The tool is designed so you can flip between different parts of a problem set. The molar mass of sodium hydroxide is 40 g mol 1. 82034 gmol 1 Appearance White deliquescent powder Odor.
324 C 615 F. B the chemical formula of the compound appears after the arrow. Worldwide production in 1998 was around 45 million tonnes.
The solute is the. It is also known as the sodium salt of hypochlorous acid HOCl. What is the mass of 356 x 1025 formula units of copper II sulfate.
We recommend you bookmark it so you can refer back to it. Here the sodium hydroxide solution is not a primary standard and is taken in a burette and a known volume of the oxalic acid a standard solution is taken in the titration flask. Worksheet Mole Conversions Name.
The molar mass of sodium hydroxide is 40 g mol 1. The molar mass of oxygen nitrogen and. Covalent compounds are composed of non-metallic elements.
As mass volume molarity molar mass then mass volume molar mass molarity. The chemical formula calculator also contains the names of a range of covalent compounds which occur as acids. Uses the formula of a reactant to determine molar mass.
Sodium hypochlorite shows following physical and chemical properties Its molar mass is 744 gmol-1. Molar Mass of Frequently Calculated Chemicals. The density of the solution is 102 g cm 3.
The A r of sodium is 23 and the A r of oxygen is 16. Find the mass concentration of H 2 SO 4 and SO 4. The molar mass of H 2 SO 4 and SO 4 is 981 g mol 1 and 961 g mol 1.
Enter formulas with proper capitalization and unpack brackets. What is the number of molecules present in 5000 g of diphosphorus pentoxide. The rates of hydrolysis by sodium hydroxide in 875 weight per cent aqueous ethanol have been measured at 0 and 30 of ethyl phenoxyacetate and the 2- and 4-fluoro chloro bromo and iodo and.
What is the mass of 125 x 1023 sodium ions. Molar Mass of Sodium carbonate Na2CO3 Molar Mass of Sodium nitrate NaNO3 Molar Mass of Sulfurous acid H2SO3 Molar Mass of Zinc sulfate ZnSO4 Bookmarking Save and Share Results. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily.
Show all work utilizing dimensional analysis wherever possible. Aluminium hydroxide AlOH 3 is used as an antacida chemical used to. A r of H 1 A r of O 16 A r of Na 23 A r of S 32 Reveal answer.
Determine the molar mass of sodium oxide Na 2 O. Sodium Hydroxide is one of the simplest hydroxides. The molarity of a sodium hydroxide solution is 051 M.
Molarity 5 12 3646 0114 moll 0114 M. Simply type in the remaining values and watch it do. 106462 View Pricing Availability.
Download Product Safety Card. Water is the solvent. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
The M r of sodium oxide is 23 2 16 62. 40 gmol Chemical Formula. Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base.
C 2 H 3 Na O 2. 8814 C 16185 F. Due to such characteristics it is often used with neutral water and acidic HCl acid to.
Determine the molar mass of calcium sulfide CaS. It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps. The molecular weight of sodium hydroxide is 40 gmol.
You can also use this molarity calculator to find the mass concentration or molar mass. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Include all units and account for significant figures.
What is the molar mass of. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Determine the molar mass of ozone.
Sodium Hydroxide chemical formula is written as NaOH. 331 K trihydrate Boiling point. Visit BYJUS for more information.
To determine the strength of the sodium hydroxide solution by titrating it against a standard solution of oxalic acid. The relative formula mass of a substance shown in grams is called one mole of that.
While adding up the atomic masses to find molecular mass do not forget to. The unit of molar mass in the SI system is kilogram per mole.
Now molecular mass calculator add all masses of substances together.

Heptane molecular mass. As separation in GC occurs in an oven at high temperatures analytes need to be volatile and thermally stable and it is therefore necessary to derivatize samples prior to analysis. 2 Names and Identifiers Expand this section. Superheated steam at 2475 C and one atmosphere.
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. The elution volumes of PEG are approximately as follows depending on the particular instrument and operating conditions. The mass of the sample containing about 6 023 1 0 23 6023 times 10 23 6 023 1 0 23 atoms or molecules see Avogadro number.
5 If all polymer chains are exactly the same then the number-average and weight-average molecular weights are exactly the same and the PDI is 1. C 10 H 22-30. Heptane is further metabolized at relatively high rates by hydroxylation before being converted to the corresponding keto forms.
C 8 H 18-57. Neopentane also called 22-dimethylpropane is a double-branched-chain alkane with five carbon atoms. Coefficents calculated by NIST from authors data.
How many elements are in the periodic table. GCMS is a commonly used platform for measuring oxidized amino acids. C 7 H 16-91.
Also important in this field is Avogadros number N. Specific Volume ft 3 lb m 3 kg 65 0405. C 9 H 20-51.
Neopentane is the simplest alkane with a quaternary carbon and has achiral tetrahedral symmetry. 16 g mol 23 g mol 1 g mol 16 g mol 40 g mol 23 g mol 1 g mol 40 g mole. 5 Related Records Expand this section.
Melting Point o C Boiling Point o C State at 25 o C. Carruth and Kobayashi 1973. 4 Spectral Information Expand this section.
Thus we put the values in the formula to find atomic mass such as. Molecular Mass Elution Volume ml 35 000 212. Temperature K A B C Reference Comment.
C 11 H 24-25. 3 Chemical and Physical Properties Expand this section. Table S10 whereas 4-aminomethylpiperidine AMP and PIP nanofilms were progressively looser with higher MWCO.
Then 15 mL of heptane and 40 mL of saturated solution of sodium chloride were added to the extract of FAMEs the mixture was shaken and phases were separated and washed subsequently with 40 mL of. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. The larger the molar-mass dispersity index the wider is the molecular weight distribution.
C 4 H 10-138-05. Assignment 1 Separation of n-Heptane from Air Stream Part 1 A process air stream at 10 atmosphere contains 131610-3 mole fraction of n-heptane. Molecular weight 2462 g mol 1 size 059 nm 3.
Octane C 9 H 20. A 9 8. In the words of Stephen Wright It doesnt matter what temperature a room is its always room temperature.
In organic chemistry an alkane or paraffin a historical trivial name that also has other meanings is an acyclic saturated hydrocarbonIn other words an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carboncarbon bonds are single. Heptane is metabolized to its parent alcohols mainly 2-heptanol and 3 heptanol and to a minor extent 1-heptanol and 4- heptanolThe heptanol metabolites are conjugated by glucuronates or sulfates and subsequently excreted in urine. The molar mass of the NaOH compound is 40 gmol.
C 2 H 6-183-89. To calculate the molecular mass sum up the atomic masses of all the elements present in the compound. Temperature K A B C Reference Comment.
Coefficents calculated by NIST from authors data. Specific Gravity air 1. Vapor pressure at 25 o C psia MNm 2 354 0244.
Amedeo Avogadro is considered to be the number of units usually molecules or atoms in a substance that is proportional to its physical mass. The ratio M w M n is called the molar-mass dispersity index 5 often called polydispersity PDI. Environmental regulations require that it be reduced to a mole fraction of 710-7.
Four major sesquiterpenoids ar-turmerone 101 α-turmerone 102 β-turmerone 103 and E-atlantone 104 with similar structures were separated from the essential oil of Curcuma longa in a single HSCCC run using a two-phase solvent system composed of n-heptaneethyl acetateacetonitrilewater 950591 vv and each compound achieved over 98 purity. C 12 H 26-10. C 3 H 8-190-42.
Air - Molecular Weight and Composition - Dry air is a mixture of gases where the average molecular weight or molar mass can be calculated by adding the weight of each component. After 2 min 5 mL of heptane and 2 mL of saturated solution of sodium chloride were added and the sample was removed from the heating block LTHS 250 Brnenska Druteva Czech Republic. Alkanes have the general chemical formula C n H 2n2The alkanes range in complexity from the simplest case of.
Nonane C 10 H 22. Specific Heat - c p - Btulb o F or calg o C JkgK 039 1675. Heptane C 8 H 18.
The basic equation mass of element mass of compound X 100. The molar mass is simply the mass of one mole of substance ie. 251 Gas chromatographyMass spectrometry GCMS.
Decane Because of the tremendous variety of possible organic compounds over six million and still counting the rules for naming structures more complex than the staight-chain alkanes are much more elaborate than those that those weve seen so far but those rules will be discussed when you take organic chemistry Molecular Masses from. Sound velocity in gas ms 216. Percent composition in chemistry typically refers to the percent each element is of the compounds total mass.
A carbon adsorption column is available to remove the heptane. C 5 H 12-130. The molecular weight range can affect many properties of.
MPD nanofilm membranes were tight with molecular weight cutoff MWCO below 246 g mol 1 based on the rejection of 6-hydroxy-2-naphthalenesulfonic acid sodium salt HNSA. In a periodic table there are a total of 118 elements. Molecular weight PEGs having molecular weight below 1000.
Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day in an ice bath or when compressed to a higher pressure. Absolute Viscosity lb m ft s centipoises 48 10-6 0007. Molecular mass or molar mass are used in stoichiometry calculations in chemistry.
Molecular weight calculator is an online tool to calculate atomic mass and molecular mass. Molecular mass calculator helps a user to complete his work in a given time without any errors. Density of liquid at atmospheric pressure lbft 3 kgm 3 375 604.
Carruth and Kobayashi 1973. C 6 H 14-95. The lower-molecular mass alkanes 1 to 4 carbons are gases at room temperature those having 5 to 20 carbons tend to be liquids with increasingly high boiling points and those with more than 20 carbons are increasingly viscous liquids and finally waxy solids at room temperature.
For instance if you had a 800 g sample of a compound that was 200 g element X and 600 g element y then the percent composition of each element would be. Benzene Gas - Specific Heat - Specific heat of Benzene Gas - C6H6 - at temperatures ranging 250 - 900 K. The tabular chart we see on the periodic table is arranged.
Subramaniam Pennathur in Methods in Enzymology 2011. In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses. It is defined to be 112 of the mass of one atom of carbon-12 and in older works is also abbreviated as amu.
10 000 228 6 000 242 4 000 251 2 000 268 1 000 284 Viscosity Apparatus The Ubbelohde suspended level viscometer shown in the. 1 Structures Expand this section.
PO 4 3-phosphate. Im glad you found your way here for chemistry help because youre in the right spot to be enlightened.
A mixture of potassium chlorate and sodium amide explodes Mellor 8258.

Sodium chlorate mass. Its structure is given below Image will be uploaded soon. SiO 3 2-silicate But note that nitrogen does not follow this pattern ie nitrate NO 3- Some nonmetals form a series of polyatomic ions with oxygen all having the same charge. Aside from the two ionic compounds originally present in the solutions AgNO 3 and NaCl two additional ionic compounds may be derived from this collection of ions.
Modification of work by the Italian voiceFlickr. Table salt also known as sodium chloride is a crystal a symmetrical solid substance made entirely of the same material. For example mixing solutions of silver nitrate and sodium chloride will yield a solution containing Ag NO 3 NO 3 Na and Cl ions.
The molar mass of KCl is 745513 gramsmol. You can see the shape of a salt crystal under a microscope and you can grow your own salt crystals for fun or for a science fair. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide.
Best Management Practices and Management Practice Survey Results PDF 62824 KB Open PDF file 33199 KB for Example Sodium Hypochlorite Purchase Specifications PDF 33199 KB. Table salt is one of the most common household chemicals. Na 3 C 6 H 5 O 7.
CHAPTER 2 1 Element Compound Mixture hydrogen magnesium oxide sea water calcium copperII sulfate honey blood mud potassium iodide. Turn ammonia solution red white or blue by. Manganese dioxide pyrolusite MnO 2 is frequently used.
The temperature necessary to effect the evolution of oxygen is reduced from. Sodium hypochlorite is a salt made up of sodium cation and hypochlorite anion. Here are the rules for using this free website.
Therefore 4 g of sodium chlorate will dissolve. Show how the energy of a chemical reaction can be given out as light by revealing how a solution of sodium chlorateI oxidises an aqueous solution of luminol 3-aminophthalhydrazide to produce a blue chemiluminescent glow without any increase in temperature. Modification of work by vxlaFlickr.
NaH 2 PO 4. 5844 gmol Chemical Formula. Although it is not itself flammable the solid product and even 30 solutions in water are powerful oxidizing agents.
Sodium chlorate appears as an odorless pale yellow to white crystalline solid. Sodium chloride MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents. Colourful reactions using ammonia solution.
Sodium hypochlorite shows following physical and chemical properties Its molar mass is 744 gmol-1. Potassium chlorate and sulfuric acid react to cause fire and possible explosions Mellor 2315. Sodium chlorate is an inorganic compound with the chemical formula Na ClO 3It is a white crystalline powder that is readily soluble in water.
NaNO 3 and AgCl. Modification of work. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
Table salt is 97 to 99 sodium chloride NaClPure sodium chloride is an ionic crystal solid. It can be crystallized as a pentahydrate NaOCl 5 H. Sodium hypochlorite commonly known in a dilute solution as bleach is a chemical compound with the formula NaOCl or NaClO comprising a sodium cation Na and a hypochlorite anion OCl or ClO It may also be viewed as the sodium salt of hypochlorous acidThe anhydrous compound is unstable and may decompose explosively.
It is hygroscopicIt decomposes above 300 C to release oxygen and leaves sodium chlorideSeveral hundred million tons are produced annually mainly for applications in bleaching pulp to produce high brightness paper. It is appreciably soluble in water and heavier so may be expected to sink and dissolve at a rapid rate. Contact with wood organic matter ammonium salts sulfur sulfuric acid various metals and.
Thermal decomposition of certain salts such as potassium chlorate or potassium nitrate. It is also known as the sodium salt of hypochlorous acid HOCl. Calcium phosphate solubility is 20 mgL and that of calcium fluoride is 16 mgL.
In association with Nuffield Foundation. Na 2 CrO 4. Sodium chloride has been used for the preparation of tris buffered saline phosphate buffered saline MPM-2 mitotic protein monoclonal 2 cell lysis buffer immunoprecipitation wash buffer LB Luria-Bertani media and dialysis buffer.
D i 53 1 C ii oThe solubility at 17 C is 7 1 g per 100 g therefore 20 7 13 g must precipitate out of the solution. 106404 View Pricing Availability. Solubility of other calcium compounds lies between the levels of these examples for example calcium arsenate 140 mgL calcium hydroxide 13 gL and calcium sulphate 27-88 gL.
Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. 0-40 25C 147. Calcium chromate solubility is 170 gL and at 0 o C calcium hypo chlorate solubility is 218 gL.
Group VA Group IVA. If a drop of a solution of sulfur dioxide in ether or alcohol is added to powdered potassium chlorate the mass explodes Mellor 2311. However other compounds are present in table salt depending on its source or additives that may be included before packaging.
The melting and boiling points of potassium chloride are 1040 K and 1690 K respectively. At 0 o C 20 o C and 100 o C the solubility of KCl in water corresponds to 2171 gL 2539 gL and 3605 gL respectively. The decomposition of potassium chlorate is catalyzed by oxides of transition metals.
Growing salt crystals is fun and easy. Answers of 13 1 g are acceptable. In its pure form sodium chloride is white.
AsO 4 3-arsenate. Its density in the solid crystalline form is 1984 grams per cubic centimetre. Open PDF file 62824 KB for Reducing Chlorate.
At 46 percent of the mass oxygen is the most plentiful element in Earths crust. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. By the way if its not actually the holidays and I havent updated this recently sorry about that.
Order EMSURE Quality Documentation. Potassium chloride is highly soluble in alcohols but not. Properties of Sodium Hypochlorite.
I promise that I wont take your. The ingredients are right in your kitchen the crystals are non-toxic and no special equipment is. Na 2 Cr 2 O 7.
It is used to increase the. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element.

Molar Mass Molecular Weight Of Bacl2 Barium Chloride Youtube
Gas constant jaxlove260 jaxlove260 10052021 Chemistry High School answered expert verified Which measure of gas does the expression nRTP represent.

Molar mass of barium chloride. Gas constant 2 See answers Avolume Advertisement Advertisement okpalawalter8 okpalawalter8 We have that the expression nRTP. Youll determine the mass percentage of every element with these masses. Barium Fe3 ironIII Al3 aluminium Cr3 ChromiumIII F-fluoride Cl-chloride Br-bromide I-iodide HO-hydroxide NO 2-nitrite NO 3-nitrate HCO 3-hydrogencarbonate O2-oxide CO 3 2-carbonate SO 4 2-sulfate HSO 4-hydrogensulfate S2-sulphide N3-nitride PO 4 3 phosphate Use the ions given above to work out the formulae of the following ionic compounds.
This process is the main. Order EMSURE Quality Documentation. This calculator converts automatically the pressure to bar with the following conversion factor.
Iron III chloride hexahydrate is used as a coagulant for sewage and industrial wastes. The melting and boiling points of potassium chloride are 1040 K and 1690 K respectively. Solution for mass of H 2 S formed part b Now that we know the limiting reagent is water this problem becomes How much H 2 S is produced from 1000 g of H 2 O and excess aluminum sulfide 1 Determine moles of 1000 g of H 2 O water.
Percentage of mass solutes mass mass of solution x 100. BaOH 2 8H 2 O. This solution will be an unsaturated aqueous solution of sodium chloride.
Its toxicity limits its applicability. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
BaIO 3 2 H 2 O. Its density in the solid crystalline form is 1984 grams per cubic centimetre. In industry barium chloride is mainly used in the purification of brine solution in caustic chlorine plants and also in the manufacture of heat treatment salts case hardening of steel.
1 psi 6894810-2 bar. Remember the compound must have a net neutral. The preparation of BaNO32 by double conversion of barium chloride and sodium nitrate or calcium nitrate is described in the Eastern European patent literature.
25 g of NaCl s will dissolve in 100 mL of water at 25C. It is antagonistic to all muscle depressants no matter whether they act primarily on nerve or muscle. Number of moles C.
Sodium chloride MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents. Barium chloride along with. Our molar mass calculator has this for a variety of other compounds.
A student reacted 102 g of barium chloride with excess silver nitrate according to. After carefully removing any sulfur formed and the heavy metals the barium nitrate is crystallized at low temperatures. Sodium chloride NaCl is a soluble salt.
0 g of liquid bromine how many grams of aluminum would we need How many milliliters of 6. It is not incorrect to divide by the larger number and express the above ratios as 10213 and 02131 respectively. Initial stimulation of contraction leads to vasoconstriction through direct action on arterial muscle peristalsis through action on smooth muscle tremors and cramps through action on the skeletal muscles and.
K_sp is called solubility product constant or simply solubility productIn general the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. It is used as a component in oil well drilling fluids. Heres an example to better demonstrate the concept.
Chloride contents rarely attain 200 molL and the highest concentrations correspond to sam- ples located close to the sea Anse des Cascades and to two samples where anthropogenic pollution is suspected Les 3 Sources and Source Canal in the Rivire des Remparts ba- sin. The atomic mass is useful in chemistry when it is paired with the mole concept. 99710928 gmol Molar mass of perchloric is acid gmol Molar mass of CaCO3 is 100.
If you do not have a water analysis you can use the values given in the right column in the input table. From the solubility table above we see that the solubility of sodium chloride is 36 g100 mL water at 25C. It is used as a radiopaque agent.
The experimental molar ratio of I_2 to Al is 1470 Note. 3856 gcm 3 anhydrous 30979 g. 106404 View Pricing Availability.
Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Barium Sulfate Structure BaSO 4 Structure Barium Sulfate BaSO 4 Uses.
Molar Mass of Ammonium aluminium sulfate AlNH4SO42 Molar Mass of Ammonium nitrate NH4NO3 Molar Mass of Barium sulfate BaSO4 Molar Mass of Calcium carbonate CaCO3. The same concept can be extended to ionic compounds and molecules. It is just a matter of preference.
Finding Molar Mass for Other Chemical Compounds. These two contaminated samples also have high con- centrations of nitrates 25-105 molL whereas nitrate. The Mass percent formula is expressed as solving for the molar mass also for the mass of every element in 1 mole of the compound.
These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. The obtained osmotic pressure with formula 2 is in psi pounds per square inch. Barium stimulates striated cardiac and smooth muscle regardless of innervation.
1000 g 18015 gmol 0555093 mol 2 Use molar ratios to determine moles of H 2 S produced from above. Number of moles C. 20823 gmol anhydrous 24426 gmol dihydrate Appearance White solid Density.
1 g of NaCl s will dissolve in 100 mL of water at 25C. Mass percent components mass total mass x 100 or. The crystals of potassium chloride are made up of face-centred cubic FCC unit cells.
5844 gmol Chemical Formula. It is used in the casting of copper anode plates as a coating material. The molar mass of KCl is 745513 gramsmol.
GoldIII Chloride B2H6 Diborane B2O3 Boron Oxide B5H9 Pentaborane BaNO32 Barium Nitrate BaOH2 Barium Hydroxide BaCl2 Barium Chloride BaCO3 Barium Carbonate BaI2 Barium Iodide BaO Barium Oxide BaO2 Barium Peroxide BaSO4 Barium Sulfate BBr3 Boron Tribromide BCl3 Boron Trichloride BeCl2 Beryllium Chloride BeI2 Beryllium Iodide BF3 Boron. Based on the chemical formula of a substance we know the composition of the substance. Since both barium chloride and barium sulfate contain one mole of barium atoms pert mole of compound the moles of barium sulfate will be the same 0100 when barium has the limiting.
Sodium chloride carbon dioxide sulfuric acid glucose. A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present in the substance. Molar mass of al2s3.
Molecular Weight Molar Mass. How do you know the Order of Elements in a Chemical Formula What is Chemical Formula. Mass Amount Half-Life Emissions percent hr kghr Air 6.
One formula unit of sodium chloride. Lets consider the saturated solution of silver chloride AgCl.
M 1 V 1 M 2 V 2 16 molL 175 mL x 1000 mL x 028 M. I Calculate the magnitude of the heat absorbed by the solution during the dissolution process assuming that the specific heat capacity of the solution is 418 Jg.
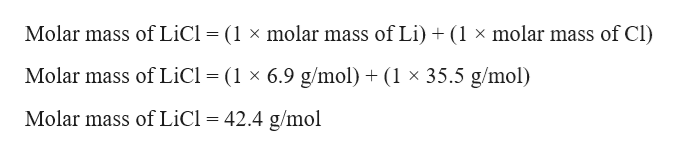
Answered Calculate The Molar Mass Of Each Bartleby
Here is a video which discusses how to calculate percent.

Molar mass of licl. Molar mass gmol Density Range of concentration. These NIR bands were assigned to the first overtone of surface hydroxyls and interlayer hydroxyls of LiOH respectively. The percent composition of the compound is.
Per unit weight there are more molecules of NH3 than of the gas mix that. NH 4 NO 3 s 2569. The volume of the solution is.
The unit asked for in the answer is mL acid. What is my theoretical yield of lithium chloride. 1295994 gmol anhydrous 23769 gmol hexahydrate Appearance yellow-brown crystals deliquescent anhydrous green crystals hexahydrate Odor.
NH 4 Brs 1678. The latter higher molecular ratio arises from the fact that the molar mass of NH3 is lower than the average for air ie. KC 2 H 3 O 2 s-1533.
This is an important distinction. NH 4 C 2 H 3 O 2 s-238. A Calculate the molar concentration of LiCl.
Do you know this. What is my percent yield. Molecular Weight Molar Mass.
Some other important properties of potassium dichromate are listed below. Anhydrous 675 g100 mL 25 C 876 g100 mL 100 C hexahydrate. The molar mass of glycine is required for this calculation and it is computed in the same fashion as its molecular mass.
The relevant given value is 730 g HCl. The molar mass of LiCl is 424 g mol 1. NaNO 3 s 2050.
If you dilute 175 mL of a 16 M solution of LiCl to 10 L determine the new concentration of the solution. 6829 g100 mL 0 C 7448 g100 mL 10 C 8425 g100 mL 25 C 887 g100 mL 40 C 12344 g100 mL 100 C Solubility. The provided mass of glycine 28 g is a bit more than one-third the molar mass 75 gmol so we would expect the computed result to be a bit.
Percent composition can be calculated the chemical formula of a compound or it can be determined experimentally. AgNO 3 s 2259. 878887 K Boiling point.
355 grams b I actually produced 6 grams of lithium chloride. Let the number moles of LiCl in 424 g mol 1 be n LiCl. The equilibrium of a saturated LiCl aqueous solution is shown below.
C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. The electrical conductivity of molten LiClKCleutSrCl2 mixtures has been measured from the liquidus temperature up to 11501220 K over the entire concentration range. The equilibrium of a saturated NaF.
What is the molar concentration of sodium ions in a 0750 M Na3PO4 solution. 605614 C 11211137 F. The molar mass of HCl 3646 gmol and the molarity of the solution 120 mol1000 mL provide the unit factors.
These NIR bands were assigned to. C 2 H 3 N. C 2 H 5 NO.
HBr HCl HClO4 KBr and NaCl are all classified as. 1274 K anhydrous 140 C hexahydrate Solubility in water. 355 gcm 3 anhydrous 192 gcm 3 hexahydrate Melting point.
Calculating the mass of Li and Cl Note. HCl HI H2SO4 LiCl and KI are all classified as. LiCl -s Li aq Cl aq At 20C the solubility of LiCl in water is 5500 gL.
Molar Mass of Frequently Calculated Chemicals. KNO 3 s 3489. C 3 H 6 O.
Acetic acid CH3CO2H formic acid HCO2H hydrofluoric acid HF ammonia NH3 and methylamine CH3NH2 are commonly classified as. For LiCl the student adds 1000 g of water initially at 150 C to a calorimeter and adds 100 g of LiCls stirring to dissolve. NaC 2 H 3 O 2 s-1732.
At a temperature of 0 o C the solubility of this compound in water corresponds to 49 grams per litre. The volume in the definition of molarity refers to the volume of the solution and not the volume of the solventThe reason for this is because one liter of solution usually contains either slightly more or slightly less than 1 liter of solvent. This compound is insoluble in alcohol and.
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. One mole of glycine C 2 H 5 O 2 N contains 2 moles of carbon 5 moles of hydrogen 2 moles of oxygen and 1 mole of nitrogen.
4239 gmol 1 Appearance white solid hygroscopic sharp Density. M Li m Li m LiCl since mass is always conserved. 1655 K Solubility in water.
K 2 Cr 2 O 7 has a crystalline reddish-orange appearance. H 2g18g x 100 111 O 16g18g x 100 889. Remember to keep the volume units consistent.
Accordingly 118 mol of LiCl will have 118 mol of Li and 118 mol of Cl. After the LiCl dissolves completely the maximum temperature reached by the solution is 356 C. LiOH KCl LiCl KOH a I began this reaction with 20 grams of lithium hydroxide.
B Calculate the molar concentration of Li and Cl-. 1382 C 2520 F. The density is estimated and the molar conductivity of these mixtures is calculated.
The molar mass of H_2O is 18 gmol The hydrogens make up 2g since each mole of hydrogen is 1g The oxygen makes up 16g. Soluble in hydrazine. For all molten mixtures the ln Λ vs 1T dependence has a nonlinear form.
Anhydrous LiOH showed two absorption bands at 7340 and 7171 cm1. Molar mass is used to describe the mass of one mole of a chemical compound. C Calculate the K sp for LiCl at 20C.
One mole of LiCl contains one mole of Li and one mole of Cl. C 2 H 4 O. Note that 1000 mL was used rather than 10 L.
LiOHH2O showed two absorption bands at 7137 and 6970 cm1. In chemistry molar concentration or molarity is defined as moles of solute per total liters of solution. We can summarize the two conversions as follows.
We select unit factors so as to cancel units. Both the specific and molar conductivities have negative. 1001 C 1834 F.
The hydration behavior of LiOH LiOHH2O and LiCl was observed by near-infrared NIR spectroscopy. 169 2 C 3 H 8 5 O 2 3 CO 2 4 H 2 O a If I start with 5 grams of C 3 H 8.
