Test cycles according to this standard usually run 168 hours. The first number under the column headed RQ is the reportable quantity in pounds.
Lead Chromate Precipitate 2 Of 3 Stock Image C036 3126 Science Photo Library
SnOH2 tin II hydroxide 28.
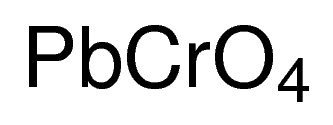
Lead 2 chromate. Similar calculation for the leadII nitrate yields. When potassium chromate is reacted with silver nitrate an insoluble white precipitate of silver chromate is formed along with potassium nitrate. Lead paint or lead-based paint is paint containing lead.
Lead reacts with air in the presence of moist and carbon dioxide forming a passivating layer. 2 lead IV sulfite 2. HPO 4 2.
AlClO33 aluminum chlorate 21. Lead sulfate PbSO 4 also known as anglesite is used in a paint pigment known as sublimed white lead. 2 If an employee is exposed to lead for more than 8 hr in any work day the permissible exposure limit as a time weighted average TWA for that day shall be reduced according to the following formula.
Lead acetate is a lead compound which has a sweet taste and is called sugar of lead An electron is a negatively charged particle that orbits the positively charged nucleus of the. SrNO32 strontium nitrate 27. IUPAC Name older name Formula.
An increase in the incidence of lung cancer has been observed among workers in industries that produce chromate and manufacture pigments containing chromate. The chemical equation for this reaction is given by. Lithium carbonate Li 2 CO 3 2510 2.
Both malleable and ductile though with little tenacity. LeadII sulfide PbS 310 28. The oxide is soluble in excess.
The uptake of hexavalent chromium compounds through the airways and digestive tract. Q 00000938 M Pb 2000050 M CrO 4 2- 469 x 10-8 Q is greater than K sp so a precipitate of leadII chromate will form. Bicarbonate HCO 3.
Magnesium ammonium phosphate MgNH 4 PO 4 25. LeadII sulfate PbSO 4 1610 8. Carcinogenic lead chromate induces DNA double-strand breaks in human lung cells.
PbSO42 lead IV sulfate 19. LeadII fluoride PbF 2 2710 8. Maximum permissible limit in ugcu m400 divided by the number of hours worked in the day.
C 2 for PbNO 3 2 00000938 M. Type 2 Chem Film Chromate Conversion Coating Tank System. 3 When respirators are used to supplement engineering and work practice controls to comply with.
Department of Defense was the first to use TCP-HF trivalent chromium hex-free and this trivalent chromate conversion coating has become such an accepted standard that a detailed specification MIL-DTL-5541 included it. Ionic bonds also melt at high temperatures. It is one of the main.
The number in parentheses is the metric equivalent in kilograms. SnCN2 tin II cyanide 18. It is easily fusible forms alloys with other metals and is an ingredient of solder and type metal.
Chromium is the proximate clastogenic species for lead chromate-induced clastogenicity in human bronchial cells. There are regulations in place banning the use of lead paint. Write the chemical formula for the following ionic compounds.
In Europe regulators are trying. Lead is added to paint to accelerate drying increase durability maintain a fresh appearance and resist moisture that causes corrosion. PbII reacts with hydroxide forming leadII oxide.
Advanced Plating Technologies no longer offers chromate services as a final finish. CuClO42 copper II chlorate 25. Testing per MIL-DTL-5541 specification.
Search by reactants AgNO 3 K 2CrO 4. Learn more about Quia. CH 3 COO C 2 H 3 O 2.
Ionic bonds are atomic bonds created by the attraction of two differently charged ionsThe bond is typically between a metal and a non-metal. Lithium phosphate Li 3 PO 4 3210 9. The chromate ion is the predominant species in alkaline solutions but dichromate can become the predominant ion in acidic solutions.
Jefferson Lab US. Very few countries have completely banned all uses of lead paint and even in the US Canada and Europe it is legal to use industrial lead paints for many applications. IO 2 4 lead IV hydrogen chromate PbHCrO 4 4 lithium peroxide Li 2O 2 cobalt II perchlorate CoC ℓO 4 2 arsenic V thiosulfate As 2S 2O 3 5 gold III fluoride AuF 3 calcium permanganate CaMnO 4 2 sodium peroxide Na 2O 2 aluminum thiocyanate Aℓ SCN 3 strontium cyanate SrOCN 2 copper.
Li 2 CO 3. Uncountable A heavy pliable inelastic metal element having a bright bluish color but easily tarnished. ChromiumVI compounds such as calcium chromate zinc chromates strontium chromate and lead chromates are highly toxic and carcinogenic in nature.
Mg3PO42 magnesium phosphate 20. The structure of the bond is rigid strong and often crystalline and solid. SnCO32 tin IV carbonate 24.
2 Pb s O 2 g 2 PbO s Reaction of lead with bases. This activity was created by a Quia Web subscriber. LeadII iodide PbI 2 7110 9.
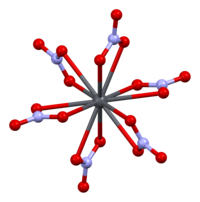
When heated in a stream of air lead burns 8. Lead chromate PbCrO 4 also known as crocoite is used to produce chrome yellow paint. Lead silicate PbSiO 3 is used to make some types of glass and in the production of rubber and paints.
These molecules are called lead compounds or sometimes lead salts. LeadII chromate PbCrO 4 2810 13. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A.
LeadII hydroxide PbOH 2 1210 15. Xie H Wise SS Holmes AL Xu B Wakeman T Pelsue SC Singh NP Wise JP. BaOH2 barium hydroxide 22.
What is the product of AgNO3 K2CrO4. Please use the below content for reference only Please use the below content for reference only Aluminum chromate conversion coatings often referred to as chemical film or under the trade names Alodine or Irridite produce a thin coating in the range of 000001-000004 inches in thickness. Lead oxide lead chromate and lead acetate are all molecules formed when lead atoms combine with atoms of other elements to form molecules.
NH42CrO4 ammonium chromate 17. As pigment leadII chromate Pb Cr O 4 chrome yellow LeadIIIV oxide Pb 3 O 4 red lead and leadII carbonate Pb C O 3 white lead are the most common forms. Cr 2 O 7 2.
Chromium III on the other hand is an essential nutritional supplement for animals and humans and has an important role in glucose metabolism. PMC free article Google Scholar 160. Lithium fluoride LiF 3810 3.
The predominance diagram shows that the position of the equilibrium depends on both pH and the analytical concentration of chromium. Lead nitrate PbNO 3 2 is used to make fireworks and other pyrotechnics. 2 AgNO3aq K2CrO4aq Ag2CrO4s 2 KNO3aq.
PbC 2 O 4. Pickerington High School Central. Atomic number 82 symbol Pb from Latin plumbum countable nautical A plummet or.
Create your own activities. In aqueous solution chromate and dichromate anions exist in a chemical equilibrium. Chromate CrO4 2-cyanide CN-dichromate Cr2O7 2-hydrogen carbonate bicarbonate HCO3-hydrogen sulfate bisulfate HSO4-hydrogen phosphate biphosphate HPO4 2-hydroxide OH-hypochlorite ClO-iodate IO3-nitrate NO3-nitrite NO2-oxalate C2O4 2-perchlorate ClO4-periodate IO4-permanganate MnO4-peroxide O2 2-phosphate PO4 3-phosphite PO3 3-silicate SiO4 4-sulfate SO4 2-sulfite SO3 2-thiocyanate SCN.
Lead dissolves slowly in cold alkalis to form plumbites. FeHSO32 iron II bisulfite 26. HgCrO4 mercury II chromate 23.
A few countries including the Philippines have regulated the lead content of both residential and industrial paints. The layer is most likely lead hydroxy carbonate 8. CrO 4 2.
Occupational exposure to lead is one of the most prevalent overexposures. 2 CrO 2 4 2 H Cr 2 O 2 7 H 2 O. Industries with high potential.
Using the initial concentrations calculate the reaction quotient Q and compare to the value of the equilibrium constant K sp. Calcium chromate chromium trioxide lead chromate strontium chromate and zinc chromate are known human carcinogens.
Lead chromate is highly corrosive and is a strong oxidizing agent. The components of alloys are ordinarily themselves metals though carbon a nonmetal is an essential constituent of steel.

7 4 Drug Discovery And Development Chemistry Libretexts
It is easily fusible forms alloys with other metals and is an ingredient of solder and type metal.

Lead compound definition. Lead is very malleable ductile and dense and is a poor conductor of electricity. In chemistry pure substances can be chemically bonded to form compounds. Alloy metallic substance composed of two or more elements as either a compound or a solution.
Known in antiquity and believed by the alchemists to be the oldest of metals lead is highly durable and resistant to corrosion. Covalent bonds and ionic bonds are two common types. Sounding lead or sounding line a line used to measure water depth.
Because it has a positive charge the metal element symbol is listed first in the chemical formula. A metallic compound is a compound that contains one or more metal elements bonded to another element. Noun When kids grow up and move out.
Compound 1 kŏm-pound kəm- kŏmpound v. Joins the verbsverb phrases with a conjunction. Soap is a compound substance.
Compound statements. Tetraethyllead or lead a gasoline additive. Learn more about alloys in this article.
The past tense of the verb lead is led not leadOne reason for the confusion might be that a similar verb read has an infinitive thats spelled the same as the past tenseBut with lead thats not how things are. Chemical formulae provide a way to represent any chemical substance using the symbol of the elements present in it. The word lead can be a component of job titles eg.
Typically the metal atom acts as the cation in the compound and is bonded to a nonmetallic anion or an ionic group. Breakthrough definition a military movement or advance all the way through and beyond an enemys frontline defense. Then that suite is executed and no other part of the if statement is executed or evaluated.
In summary a compound predicate. Tin was often compounded with lead to make pewter. Only trace elements may remain.
Lead absorption is influenced by factors such as age and physiological status. Lead dioxide can be produced by anodic oxidation of solutions of lead salts or commercially by the oxidation of red lead Pb3O4 in alkaline slurry with chlorine. It bears a negative or a positive charge a negative ion is known as anion and a positive ion is known as a cation.
This substance is used in printing inks paints and to color vinyl rubber and paper. Atomic number 82 symbol Pb from Latin plumbum countable nautical A plummet or. What does independence mean.
To combine so as to form a whole. There is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. Verb to guide on a way especially by going in advance.
The hydrogen is bonded to the oxygen. Here are examples with the verbs to speak and to present Sean spoke and. An ion is formed through removal of electrons or additions of electrons from molecules or neutral atoms by the combination with other particles.
A mixture is not a compound. The type of bond keeping elements in a compound together may vary. Lead chromate primarily affects the lungs causing shortness of.
What are compound predicates. The periodic table is a tabular arrangement of the chemical elements. Kirk-Othmer Encyclopedia of Chemical Technology 1999-2016.
To produce or create by combining two or more ingredients or parts. If the substances do not chemically bond they form mixtures. Is a part of an independent clause.
Carr DS et al. Chemical Compound Formulas. Lead a soft silvery white or grayish metal in Group 14 IVa of the periodic table.
A compound is a material formed by chemically bonding two or more chemical elements. Diodes definition of products with a Lead Free Finish These products will have no external Lead but will still contain internal Lead in the form of high temperature die bonding solder. The elements are always present in fixed ratios in any compound.
Compound means a substance formed as a blend of various elements chemically in a certain proportion by weight. Exposure to lead occurs mainly via inhalation of lead-contaminated dust particles or aerosols and ingestion of lead-contaminated food water and paints 173 174. A compound predicate contains more than one verb.
It is entirely new substance which possesses properties different from that of its constituent substances. An ion is basically an atom or a group of atoms in which the number of electrons is not equal to the number of protons. Carbon dioxide is another example of a homogeneous compound.
Both malleable and ductile though with little tenacity. To serve as a channel for. Lead Chromate is a yellow orange or red colored crystalline inorganic compound that emits toxic chromium fumes upon heating.
Provided below is a list of the chemical formulas of some common chemical compounds along with their molecular weights. Led is the correct way to spell the past tense of lead. Lead Pb Platinum Pt Frequently Asked Questions FAQs.
If all expressions are false the suite of the else clause if present is executed. Contains one subject and multiple verbsverb phrases. What does compound mean.
Uncountable A heavy pliable inelastic metal element having a bright bluish color but easily tarnished. A compound predicate can include any variation of the predicates described above except simple predicate so long as it contains more than one verb. The definition of compound predicate is a predicate that has two or more verbs or verb phrases.
Diodes definition of Lead Free Diodes Incs products defined as Lead Free will contain no purposefully added Lead either internally or externally. For example Water salt carbon dioxide sodium chloride etc. Lead engineer or Marketing lead where it indicates seniority or a.
The compound is the unification of various elements so that the atoms present in. To direct on a course or in a direction. Compose or make up.
Definition of Compound. These lead compounds undergo more extensive optimization in a subsequent step of drug discovery called lead optimization LO. Hit to lead H2L also known as lead generation is a stage in early drug discovery where small molecule hits from a high throughput screen HTS are evaluated and undergo limited optimization to identify promising lead compounds.
Compounded compounding compounds vtr. Adults absorb 35 to 50 of lead through drinking water and the absorption rate for children may be greater than 50. See section Boolean operations for the definition of true and false.
The definition of independence is freedom from the control or influence of others. However the air you are breathing. An example of a homogeneous compound is pure water H 2 O.
It is organized in order of increasing atomic number. Lead compound a chemical compound in drug discovery not necessarily with lead the metal Leadlag compensator a component in a control system. In the human body the greatest.
Compound definition composed of two or more parts elements or ingredients. The while statement The while statement is used for repeated execution as long as an expression is true. To settle a debt for.
Urea on the other hand has an NPK grade of 46-0-0 making it more economical to transport. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator.

Equation For Pb No3 2 H2o Lead Ii Nitrate Water Youtube
The legal limit for a contaminant reflects the level that protects human health and that water systems can achieve using the best available technology.
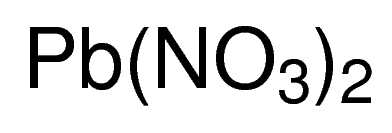
Lead nitrate formula. Iron II chloride 18. NH42SO4 ammonium sulfate 20. Example- PbNO 3 4 write the name lead nitrate.
AC193320000 AC193320100 AC193320500 CAS-No 10099-74-8 Synonyms Nitric acid lead2 salt. In its solid form silver nitrate is coordinated in a trigonal planar arrangement. These crystals are rhombohedral in shape but when they are subjected to temperatures above 32 degrees.
Salts containing this ion are called nitrates. Ionic Compounds Naming and Formula Writing. For example the compound magnesium nitrate is written as MgNO 3 2.
Public water systems are still required to ensure compliance with all Federal and State Safe Drinking Water regulations at the posted taps. 13 potassium fluoride is KF 14 ammonium sulfate is NH 42SO 4 15 magnesium iodide is MgI 2 16 copper II sulfite is CuSO 3 17 aluminum phosphate is AlPO 4 18 lead II nitrite is PbNO 22. Ninety percent of synthetic urea produced now is for fertilizers.
Lead IV dichromate PbCr2O72 7. ONE-SCHOOLNET Periodic Table. Sodium carbonate Na2CO3 6.
Chemical formula plays an important role in understanding different concepts of chemistry. It is often used as a precursor to other silver-containing compounds. The transfer of electrons between metals and non-metals produces charged particles called ions.
Since lead has more than one oxidation state we must figure out which lead we have. Copy this to my account. 10 MnNO 33 is manganese III nitrate 11 FePO 4 is iron III phosphate 12 CoCO 3 is cobalt II carbonate Name to formula problems.
Ammonium nitrate N2H4O3 is one such compound and has an NPK rating of 34-0-0. Strontium acetate SrC2H3O22 3. H 2 CO 3.
If the formula is given write down the name and if the name is given write down the formula. Nitrate is a polyatomic ion with the chemical formula NO 3. So our roman numeral will be IV.
LeadII nitrate is an inorganic compound with the chemical formula PbNO 3 2. Sodium chloride NaCl and magnesium oxide MgO. KNO3 potassium nitrate 8.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Drinking cooking making baby formula. Other chemical compounds have been used as popular fertilizers over the last century.
The entire group 1 metal can react with oxygen to form metal oxide. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Lead dinitrate Recommended Use Laboratory chemicals.
It is used in making photographic films and in laboratory setting as a staining agent in protein visualization in PAGE. Ammonium cyanide NH4CN 5. Product Name LeadII nitrate Cat No.
For example the symbol for lead Pb comes from the Latin word. In room temperature ammonium nitrate appears in a white crystalline form and it is also colorless. Its melting point is at 1696 degrees Celsius or 3373 degrees Fahrenheit.
SrNO32 strontium nitrate 27. SnOH2 tin II hydroxide 28. E-mail to a friend.
The two charges balance in a 1. Ammonium nitrate is a chemical compound with the formula NH4NO3. Nitrates are common components of fertilizers and explosives.
MgOH2 magnesium hydroxide 9. Since each nitrate 4 of them has a 1- charge the Pb must be 4. Na Mg 2 Non.
Silver nitrate is an inorganic compound with the chemical formula AgNO3. In lead II iodide the charges balance in a 1. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another.
E-mail to a friend. As a general rule you should know the names and symbols for elements 1-36. Nitrate medications prescribed for people who have high blood pressure or heart disease dilate blood vessels easing the resistance to blood flowing through them.
Reaction of Group 1 Elements. Uses advised against Food drug pesticide or biocidal product use. Lead II chlorate PbClO32 2.
The ion is the conjugate base of nitric acid. LiCIO3 lithium chlorate 10. Zinc phosphate Zn3PO42 4.
Known since the Middle Ages by the name plumbum dulce the production of leadII nitrate from either metallic lead or lead oxide in nitric acid was small-scale for direct use in making other lead. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. In this example the compound has one magnesium atom and two.
PbNO 3 4 is named leadIV nitrate Highlight to reveal names. Reaction with Oxygen. The Sink for Dishwashing Only sign may be used by facilities to indicate to customers that the tap is not to be used for other human consumption eg.
Ammonium nitrate formula NH 4NO 3 90. 2 ratio so the formula is PbI_2. An ionic compound is composed of a metal and a non-metal.
EPA sets legal limits on over 90 contaminants in drinking water. Cu2CO3 Section B Write the formula of the ionic compounds containing polyatomic ions 1. In addition you should know names and symbols for all elements in Groups I and II as well as the halogens Group VII noble gases Group VIII and some other metals used frequently.
Chemical formulas can be. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. NiS nickel II sulfide Write the chemical formula for each of the following compounds.
Ionic Compounds Naming and Formula Writing. Na2CrO4 sodium chromate 19. It commonly occurs as a colourless crystal or white powder and unlike most other leadII salts is soluble in water.
To balance the charges we require two nitrate ions per lead II ion and so lead II nitrate is PbNO_3_2. Details of the supplier of the safety data sheet Emergency Telephone Number For information US call. Ammonium phosphate formula NH 4 3 PO 4.
1 ratio so potassium iodide is simply KI. Use these pages as a study guide. Copy this to my account.
The nitrate ion with the partial charges shown. An example of an insoluble nitrate is bismuth oxynitrate. It is composed of nitric acid and salt of ammonia.
Metals lose electrons to produce positve ions called cations. This very benefit however is the second important mechanism through which nitrates and nitrites cause adverse effects. Na2S sodium sulfide 17.
The potassium ion is K and the iodide ion is I-. More complex molecules like the nitrate ion NO 3 will be enclosed in parentheses if more than one occurs in the formula and the subscript will be placed outside the closing parenthesis. Learn about the health effects of lead who is at risk how to test for lead in paint or other areas of your home how to find or become a lead-safe certified firm and more about the Lead Renovation Repair and Painting RRP rule.
PbCl2 lead II chloride 7. Excessive dilation of blood vessels can lead to a dangerous drop in blood pressure. Carp Cyprinus carpio were held for 48 hr in lead nitrate concn of between 0 and 20 mg leadL with and without one of the three complexans EDTA NTA or DTPAThe accumulation of lead in both viscera and gills was dose-related with the highest levels for viscera and gills being 86000 and 4560 mgkg dry.
Almost all inorganic nitrates are soluble in water.
Lead absorption is influenced by factors such as age and physiological status. Lead Chromate is a yellow orange or red colored crystalline inorganic compound that emits toxic chromium fumes upon heating.

Pdf Precipitation Of Lead Ii Chromate Folk Narongrit Academia Edu
The less water soluble the chromate the higher was its elimination via the feces.

Lead chromate in water. It is expected that chromate use in open recirculating cooling systems will be banned altogether by the end of 1993. Uncountable A heavy pliable inelastic metal element having a bright bluish color but easily tarnished. In the human body the greatest.
First determine the overall and the net-ionic equations for the reaction that. Pb2aq 2NO3-aq 2Kaq 2I-aq. LeadII is precipitated by chromate under acidic conditions.
Answer 1 of 4. 815 x 10-4. The membrane has a tight pore structure that effectively removes up to 99 of all contaminants and impurities such as total dissolved solids chemicals.
When used as oxidizing agents or titrants in a redox. Lead chromate primarily affects the lungs causing shortness of. It is easily fusible forms alloys with other metals and is an ingredient of solder and type metal.
ChromiumVI compounds such as calcium chromate zinc chromates strontium chromate and lead chromates are highly toxic and carcinogenic in nature. Lead reacts vigorously with fluorine F 2 at room temperature and chlorine Cl 2 when heated forming the corresponding leadII halides 8. Both malleable and ductile though with little tenacity.
As an Anti-knock Agent Tetraethyl lead is utilized as an anti-knock. 271 synonyms for lead. Li 2 CO 3.
This substance is used in printing inks paints and to color vinyl rubber and paper. Atomic number 82 symbol Pb from Latin plumbum countable nautical A plummet or. Class 1A chem film is thicker and darker than the lighter thinner Class 3 chem film.
The reaction is as follows. Chromate and dichromate salts of heavy metals lanthanides and alkaline earth metals are only very slightly soluble in water and are thus used as pigments. Guide to watercolor pigments.
Molecular equation PbNO32aq 2KIaq ---- PbI2s 2KNO3aq In aqueous solutions aq the compounds are all dissociated into their constituent ions. Chromate salts contain the chromate anion. In Europe regulators are trying to ban paint ingredients containing lead on a chemical-by-chemical basis and have banned the use of lead chromate pigments.
Synonyms for lead in Free Thesaurus. This insoluble solid is the precipitate and it has a red-brown color. This characteristic provides.
Lead is added to paint to accelerate drying increase durability maintain a fresh appearance and resist moisture that causes corrosion. Aluminum chromate conversion coatings often referred to as chemical film or under the trade names Alodine or Irridite produce a thin coating in the range of 000001-000004 inches in thickness. Calcium chromate solubility is 170 gL.
When inhaled hexavalent chrome is a suspected carcinogen. Therefore as of May 1990 the use of chromate in comfort cooling towers was banned by the EPA. Lead paint or lead-based paint is paint containing leadAs pigment leadII chromate Pb Cr O 4 chrome yellow LeadIIIV oxide Pb 3 O 4 red lead and leadII carbonate Pb C O 3 white lead are the most common forms.
The legal limit for a contaminant reflects the level that protects human health and that water systems can achieve using the best available technology. When silver nitrate and potassium chromate solutions are mixed together then there is formation of silver chromate and potassium nitrate. When one takes up large amounts of calcium.
Chromium III on the other hand is an essential nutritional supplement for animals and humans and has an important role in glucose metabolism. What color is the precipitate between silver nitrate and potassium chromate. Pb s F 2 g PbF 2 s Pb s Cl 2 g PbCl 2 s LeadII is precipitated by chloride bromide and.
Please place orders before the 5th of December to ensure delivery. The class of the chemical film will also have an effect on the chromate finish. Aluminum chromate conversion coatings are amorphous in structure with a gel-like composition hydrated with water.
The Complete ionic equation shows all the ions present in solution separately. PbC 2 O 4. Pb 2 aq CrO 4 2 aq PbCrO 4 s yellow Reaction of lead with halogens.
This prevents lead from dissolving in drinking water and thereby prevents it from entering the human body. Efforts to restrict the use of lead paint date back to the 1920s but it was not banned for residential use in the US. Exposure to lead occurs mainly via inhalation of lead-contaminated dust particles or aerosols and ingestion of lead-contaminated food water and paints 173 174.
Lead chromate is highly corrosive and is a strong oxidizing agent. The uptake of hexavalent chromium compounds through the airways and digestive tract. As the silver nitrate solution is slowly added a precipitate of silver chloride.
Improper acid activation or pH levels in tanks can. EPA sets legal limits on over 90 contaminants in drinking water. Absorbed chromium was retained in the spleen and bone marrow in all three cases and also in the liver and.
Will a precipitate of leadII chromate form. The lead-containing pigment chrome yellow was used for a very long time before environmental regulations discouraged its use. Calcium carbonate has a positive effect on lead water pipes because it forms a protective leadIIcarbonate coating.
Adults absorb 35 to 50 of lead through drinking water and the absorption rate for children may be greater than 50. 51Chromium labelled sodium zinc and lead chromates were studied. Reverse Osmosis commonly abbreviated to RO is a water treatment method traditionally known for removing salt from seawater it is also used to purify drinking water by forcing untreated water molecules through a semipermeable membrane.
EPA rules also set water-testing. As Soldering Material Due to its low melting temperature and wide availability lead along with tin and other alloys act as the most commonly used solder material for electronics. Watertest Systems will be closed from December 23rd until January 4th.
Calcium phosphate solubility is 20 mgL and that of calcium fluoride is 16 mgL. What are synonyms for lead. Ineffective rinses can lead to contaminated tanks.
The preparation stages of chromate conversion coatings are important too. 250 mL of 00020 M potassium chromate are mixed with 750 mL of 0000125 M leadII nitrate. Go in front of head be in front be at the head of walk in front of guide conduct steer escort precede usher pilot.
The colored linksat the top of the screen take you to detailed information on modern watercolor pigments based on evaluations of over 750 commercial watercolor paints the most comprehensive watercolor paint information available on the InternetIf you dont see the color links click here The information on this site is built around a simple strategyfor. Table 1173 - Reportable Quantities of Hazardous Substances Designated Pursuant to Section 311 of the Clean Water Act. Sodium chromate and the less soluble zinc chromate were absorbed into the blood resulting in increased urinary excretion of chromium.
K sp of leadII chromate is 18 x 10-14. The most recent concern relating to chromate treatment involves chromate present in cooling tower drift. White lead lead sulfate and lead chromate are used as coloring elements in paints and ceramic glazes notably in the colors red and yellow.
Its preparation is an entertaining and popular. Learn about the health effects of lead who is at risk how to test for lead in paint or other areas of your home how to find or become a lead-safe certified firm and more about the Lead Renovation Repair and Painting RRP rule.
Contact may irritate skin eyes and mucous membranes.
Lead ii nitrate. The lead is in the 2 oxidation state. Ionic bonds also melt at high temperatures. Ingested nitrate or nitrite under conditions that result in endogenous nitrosation which could lead to the formation of known carcinogens such as N-nitroso compounds IARC 2010.
A Both the Assertion and the Reason are correct and the Reason is the correct explanation of the Assertion. Zinc and Lead II nitrate react to form Zinc Nitrate and Lead. Copper I sulfite Cu2SO3 8.
Zinc phosphate Zn3PO42 4. Cest un cristal incolore ou une poudre blanche et un oxydant stable et fort. LeadII nitrate Revision Date 19-Jan-2018 Eye Contact Rinse immediately with plenty of water also under the eyelids for at least 15 minutes.
Aluminum bins and wooden bins protected against impregnation by ammonium nitrate are permissible. It is one of the few lead compounds that can dissolve in water. Depuis le XX e siècle il est utilisé.
At room temperature it is a bright yellow odorless crystalline solid that becomes orange and red when heated. 2 ratio so the formula is PbI_2. Calculate the grams of leadII iodide that can be produced from 500 moles of potassium iodide.
There are a number of ions that are not individual atoms but are composed of multiple atoms that are covalently bonded together. CID 944 Nitric acid CID 5352425 Lead Dates. Strontium acetate SrC2H3O22 3.
Since each nitrate 4 of them has a 1- charge the Pb must be 4. Since lead has more than one oxidation state we must figure out which lead we have. Lead sulfate appears as a white crystalline solid.
LeadII acetate is the other lead compound that can dissolve. Sulfuric acid lead2 salt 11 More. Contrairement à dautres sels de plombII il est soluble dans leau.
The reaction between lead nitrate and potassium iodide is an example of a precipitation reaction. To balance the charges we require two nitrate ions per lead II ion and so lead II nitrate is PbNO_3_2. Sodium Phosphate and Calcium Chloride react to form Calcium Phosphate and Sodium Chloride.
If large quantities of the. 2AlBr 3 3 Cl 2 2AlCl 3 3Br 2 Single Replacement 3. LeadII nitrate is an inorganic compound with the chemical formula PbNO 3 2.
Do not use mouth-to-mouth method if victim ingested or inhaled the. Cu2CO3 Section B Write the formula of the ionic compounds containing polyatomic ions 1. Lead nitrate is a chemical compound.
Steps to balance chemical equation. SnOH2 tin II hydroxide 28. The compound currently has a few specialized applications such as the manufacture of solar cells and X-ray and gamma-ray detectors.
Zn PbNO 3 2 ZnNO 3 2 Pb Single Replacement 2. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. The structure of the bond is rigid strong and often crystalline and solid.
Write the equation for this reaction then balance the equation. If you dissolve leadII nitrate and potassium iodide in water they will react to form leadII iodide and potassium nitrate. Write the Unbalanced Chemical Equation.
It is noncombustible but it will accelerate the burning of combustible materials. Insoluble in water and sinks in water. Following are the easy steps to balance the equation.
Lead nitrate PbNO32 More. Son usage principal depuis le Moyen Âge sous le nom de plumb dulcis a été comme matière première de nombreux pigments. The International Agency for Research on Cancer IARC classifies nitrates and nitrites as probably carcinogenic to humans Group 2A under certain conditions ie.
It was known long ago as plumb dulcis. The two charges balance in a 1. A lead nitrate on thermal decomposition gives lead oxide brown coloured nitrogen dioxide and oxygen gas.
It commonly occurs as a colourless crystal or white powder and unlike most other leadII salts is soluble in water. 10 MnNO 33 is manganese III nitrate 11 FePO 4 is iron III phosphate 12 CoCO 3 is cobalt II carbonate Name to formula problems. When these two solutions are mixed leadII bromide PbBr_2 an insoluble ionic compound and sodium nitrate NaNO_3 another soluble ionic compound will be formed.
Aluminum Bromide and Chlorine gas react to form Aluminum Chloride and Bromine gas. Le nitrate de plombII est un sel inorganique de plomb et dacide nitrique. Sodium carbonate Na2CO3 6.
However this group of atoms is most stable when it has either lost of gained an electron and thus existed as a charged ion. It is toxic. 2Na 3 PO 4 3 CaCl 2 6NaCl Ca 3 PO 4 2.
Let us consider an. Inhalation Remove to fresh air. A Write a balanced equation for the reaction between aqueous leadII nitrate and aqueous potassium chloride to form solid leadII chloride and aqueous potassium nitrateExpress your answer as a.
The complete ionic equation will look like this. CID 1118 Sulfuric acid CID 5352425 Lead Dates. Ionic bonds are atomic bonds created by the attraction of two differently charged ionsThe bond is typically between a metal and a non-metal.
The partitions dividing. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. The material is soluble in water.
1 ratio so potassium iodide is simply KI. LeadII chloride can be made as a white precipitate by adding a solution containing chloride ions to leadII nitrate solution. It was formerly called plumbous iodide.
PbNO 3 4 is named leadIV nitrate Highlight to reveal names. Ammonium cyanide NH4CN 5. So our roman numeral will be IV.
May be mildly. 13 potassium fluoride is KF 14 ammonium sulfate is NH 42SO 4 15 magnesium iodide is MgI 2 16 copper II sulfite is CuSO 3 17 aluminum phosphate is AlPO 4 18 lead II nitrite is PbNO 22. Lead IV dichromate PbCr2O72 7.
In lead II iodide the charges balance in a 1. Calculate the grams of leadII iodide that can be produced from 7500 grams of potassium iodide. Lead II chlorate PbClO32 2.
Known since the Middle Ages by the name plumbum dulce the production of leadII nitrate from either metallic lead or lead oxide in nitric acid was small-scale for direct use in making other lead. The potassium ion is K and the iodide ion is I-. Lead nitrate reacts with potassium iodide to form yellow ppt of lead iodide and the reaction is double displacement as well as precipitation reaction.
PbNO3 2 2KI PbI 2 2KNO 3. It has lead and nitrate ions in it. SrNO32 strontium nitrate 27.
Skin Contact Wash off immediately with plenty of water for at least 15 minutes. Example- PbNO 3 4 write the name lead nitrate. LeadII iodide or lead iodide is a salt with the formula PbI 2.
It crackles when it is heated. Lead nitrate is a white crystalline solid. 1910109i4iib Due to the corrosive and reactive properties of ammonium nitrate and to avoid contamination galvanized iron copper lead and zinc shall not be used in a bin construction unless suitably protected.
It is an oxidizing agent. You could use things like sodium chloride solution to provide the chloride ions but it is usually easier just to add some dilute hydrochloric acid. Lead nitrate is a white solid.
Reaction between lead nitrate and potassium iodide. Cadmium phosphate Cd3PO42 9.