Urea on the other hand has an NPK grade of 46-0-0 making it more economical to transport. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator.

Equation For Pb No3 2 H2o Lead Ii Nitrate Water Youtube
The legal limit for a contaminant reflects the level that protects human health and that water systems can achieve using the best available technology.
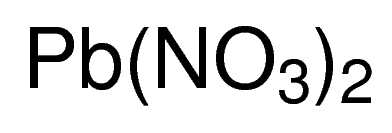
Lead nitrate formula. Iron II chloride 18. NH42SO4 ammonium sulfate 20. Example- PbNO 3 4 write the name lead nitrate.
AC193320000 AC193320100 AC193320500 CAS-No 10099-74-8 Synonyms Nitric acid lead2 salt. In its solid form silver nitrate is coordinated in a trigonal planar arrangement. These crystals are rhombohedral in shape but when they are subjected to temperatures above 32 degrees.
Salts containing this ion are called nitrates. Ionic Compounds Naming and Formula Writing. For example the compound magnesium nitrate is written as MgNO 3 2.
Public water systems are still required to ensure compliance with all Federal and State Safe Drinking Water regulations at the posted taps. 13 potassium fluoride is KF 14 ammonium sulfate is NH 42SO 4 15 magnesium iodide is MgI 2 16 copper II sulfite is CuSO 3 17 aluminum phosphate is AlPO 4 18 lead II nitrite is PbNO 22. Ninety percent of synthetic urea produced now is for fertilizers.
Lead IV dichromate PbCr2O72 7. ONE-SCHOOLNET Periodic Table. Sodium carbonate Na2CO3 6.
Chemical formula plays an important role in understanding different concepts of chemistry. It is often used as a precursor to other silver-containing compounds. The transfer of electrons between metals and non-metals produces charged particles called ions.
Since lead has more than one oxidation state we must figure out which lead we have. Copy this to my account. 10 MnNO 33 is manganese III nitrate 11 FePO 4 is iron III phosphate 12 CoCO 3 is cobalt II carbonate Name to formula problems.
Ammonium nitrate N2H4O3 is one such compound and has an NPK rating of 34-0-0. Strontium acetate SrC2H3O22 3. H 2 CO 3.
If the formula is given write down the name and if the name is given write down the formula. Nitrate is a polyatomic ion with the chemical formula NO 3. So our roman numeral will be IV.
LeadII nitrate is an inorganic compound with the chemical formula PbNO 3 2. Sodium chloride NaCl and magnesium oxide MgO. KNO3 potassium nitrate 8.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Drinking cooking making baby formula. Other chemical compounds have been used as popular fertilizers over the last century.
The entire group 1 metal can react with oxygen to form metal oxide. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Lead dinitrate Recommended Use Laboratory chemicals.
It is used in making photographic films and in laboratory setting as a staining agent in protein visualization in PAGE. Ammonium cyanide NH4CN 5. Product Name LeadII nitrate Cat No.
For example the symbol for lead Pb comes from the Latin word. In room temperature ammonium nitrate appears in a white crystalline form and it is also colorless. Its melting point is at 1696 degrees Celsius or 3373 degrees Fahrenheit.
SrNO32 strontium nitrate 27. SnOH2 tin II hydroxide 28. E-mail to a friend.
The two charges balance in a 1. Ammonium nitrate is a chemical compound with the formula NH4NO3. Nitrates are common components of fertilizers and explosives.
MgOH2 magnesium hydroxide 9. Since each nitrate 4 of them has a 1- charge the Pb must be 4. Na Mg 2 Non.
Silver nitrate is an inorganic compound with the chemical formula AgNO3. In lead II iodide the charges balance in a 1. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another.
E-mail to a friend. As a general rule you should know the names and symbols for elements 1-36. Nitrate medications prescribed for people who have high blood pressure or heart disease dilate blood vessels easing the resistance to blood flowing through them.
Reaction of Group 1 Elements. Uses advised against Food drug pesticide or biocidal product use. Lead II chlorate PbClO32 2.
The ion is the conjugate base of nitric acid. LiCIO3 lithium chlorate 10. Zinc phosphate Zn3PO42 4.
Known since the Middle Ages by the name plumbum dulce the production of leadII nitrate from either metallic lead or lead oxide in nitric acid was small-scale for direct use in making other lead. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. In this example the compound has one magnesium atom and two.
PbNO 3 4 is named leadIV nitrate Highlight to reveal names. Reaction with Oxygen. The Sink for Dishwashing Only sign may be used by facilities to indicate to customers that the tap is not to be used for other human consumption eg.
Ammonium nitrate formula NH 4NO 3 90. 2 ratio so the formula is PbI_2. An ionic compound is composed of a metal and a non-metal.
EPA sets legal limits on over 90 contaminants in drinking water. Cu2CO3 Section B Write the formula of the ionic compounds containing polyatomic ions 1. In addition you should know names and symbols for all elements in Groups I and II as well as the halogens Group VII noble gases Group VIII and some other metals used frequently.
Chemical formulas can be. Chemistry is all about learning chemical elements and compounds and how these things work together to form several chemical equations that are hard to understand. NiS nickel II sulfide Write the chemical formula for each of the following compounds.
Ionic Compounds Naming and Formula Writing. Na2CrO4 sodium chromate 19. It commonly occurs as a colourless crystal or white powder and unlike most other leadII salts is soluble in water.
To balance the charges we require two nitrate ions per lead II ion and so lead II nitrate is PbNO_3_2. Details of the supplier of the safety data sheet Emergency Telephone Number For information US call. Ammonium phosphate formula NH 4 3 PO 4.
1 ratio so potassium iodide is simply KI. Use these pages as a study guide. Copy this to my account.
The nitrate ion with the partial charges shown. An example of an insoluble nitrate is bismuth oxynitrate. It is composed of nitric acid and salt of ammonia.
Metals lose electrons to produce positve ions called cations. This very benefit however is the second important mechanism through which nitrates and nitrites cause adverse effects. Na2S sodium sulfide 17.
The potassium ion is K and the iodide ion is I-. More complex molecules like the nitrate ion NO 3 will be enclosed in parentheses if more than one occurs in the formula and the subscript will be placed outside the closing parenthesis. Learn about the health effects of lead who is at risk how to test for lead in paint or other areas of your home how to find or become a lead-safe certified firm and more about the Lead Renovation Repair and Painting RRP rule.
PbCl2 lead II chloride 7. Excessive dilation of blood vessels can lead to a dangerous drop in blood pressure. Carp Cyprinus carpio were held for 48 hr in lead nitrate concn of between 0 and 20 mg leadL with and without one of the three complexans EDTA NTA or DTPAThe accumulation of lead in both viscera and gills was dose-related with the highest levels for viscera and gills being 86000 and 4560 mgkg dry.
Almost all inorganic nitrates are soluble in water.
Chemical Product and Company Identification Product Name. Potassium nitrate sulfur and carbon Gunpowder Oxygen and water Sea foam.
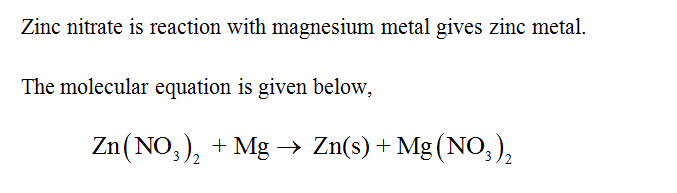
Answered A Piece Of Magnesium Metal Is Dropped Bartleby
Phosphorus is used in photosynthesis and overall.

Magnesium & zinc nitrate. Magnesium a mineral that supports immune heart. Zinc oxide is an inorganic compound with the formula Zn OZnO is a white powder that is insoluble in water. The soil does not need to.
Therefore none of zinc copper or lead will displace magnesium ions from solution so that there will be no reaction. 02ppm DTPA extractable Cu Designed and Developed by National Informatics Centre Information and data in this application is managed by State Agricultural Departments and Department of Agriculture and Farmers Welfare STCR. Cium Ca magnesium Mg boron B chlorine Cl copper Cu iron Fe manganese Mn molybdenum Mo nickel Ni and zinc Zn.
These 17 essential elements also called nutrients are often split into three groups fig. The negative ion anion is written second in the name. Nitrogen and sulfur are essential to the supply of amino acids and proteins.
Potassium nitrate may increase the hyperkalemic activities of Magnesium sulfate. It is used as an additive in numerous materials and products including cosmetics food supplements rubbers plastics ceramics glass cement lubricants paints ointments adhesives sealants pigments foods batteries ferrites fire retardants and first-aid tapes. 06 ppm DTPA extractable Zn Boron.
Polyatomic ions are ions which consist of more than one atom. The authors noted that the ability of beetroot juice to lower blood pressure depends on the nitrate. The essential nutrients used include calcium nitrate potassium sulphate potassium nitrate mono potassium phosphate and magnesium sulphate.
Isnt it interesting to consider how science is all around us. Zinc Acetate is made from zinc nitrate and acetic anhydride. Hydrogen forms water by combining with the oxygen.
Magnesium MSDS Section 1. Zn 3 PO 4 2. Zinc copper and lead do not react with magnesium nitrate because in the activity sequence magnesium is higher because it is more reactive than other metals.
This form may aid in reducing the duration of the common cold JRSM Open. TBHQ Tertiary Butyl Hydro Quinone. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atoms.
The best way to learn new. Zinc which promotes wound. Magnesium is the only metallic component of chlorophyll.
20ppm DTPA extractable Mn Copper. This is our newest publication and has been created to support the school technician profession in Scotland. 05 ppm hot water soluble boron Iron.
Mangafodipir may decrease the excretion rate of Potassium nitrate which could result in a higher serum level. Potassium nitrate enriched with micronutrients. Magnesium ribbons turnings or sticks Chemical Name.
Zinc acetate is another chemically-altered form of zinc and considered to be more absorbable than gluconate. Potassium nitrate enriched with Zinc. Magnesium trisilicate may decrease the excretion rate of Potassium nitrate which could result in a higher serum level.
Magnesium is a chemical element with the symbol Mg and atomic number 12. Zinc picolinate and zinc gluconate are forms of dietary supplements that we use to prevent zinc deficiency. ZINC ZIRCONIUM finer than 100 mesh.
SLM4408 SLM2263 SLM3637 CAS. Its in the food we eat and the air we breathe. The atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit.
Potassium nitrate enriched with Magnesium. CHARCOAL AND LAMPBLACK any mesh size The following ignition material is restricted to 25 ft per. Potassium Nitrate enriched with Boron.
DC Directly Compressible Calcium Carbonate. Zinc aluminum magnesium and copper Zamak Advertisement Science Is All Around Us. Finally there are seven elements that plants need in tiny amounts.
TITANIUM ALLOYS all are coarser than 100 mesh MAGNESIUM AND MAGNESIUM ALLOY S 10 to 100 mesh with or without 50 Mg chips ALUMINUM POWDERS 100 mesh or coarser. 45ppm DTPA extractable Fe Manganese. 2X DMSO 999 Pharma Grade No Odor - Dimethyl.
Adding Epsom salts diluted to 85 oz. Amyl Nitrate Strange Connection Dirty Bastard Remix by Adriano. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website.
Magnesium deficiency can be misidentified as zinc or chlorine deficiency a virus or as natural aging so notice the details on your plants and cross-examine it with the symptoms on this list. And the more we experiment with it the more we uncover various truths about it. Without it plants cant process sunlight.
To learn more about chemical formula nomenclature of organic compounds and other topics related to chemical formula in chemistry download BYJUS The Learning App. Potassium nitrate enriched with Sulfate. All group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.
It is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column group 2 or alkaline earth metals of the periodic table. Healthy soil that is high in organic matter usually contains adequate amounts of each of these micronutrients. Lead Lux Magnesium Manganese Molybdenum Nickel Nitrate Nitrite Nitrogen ORP Ozone pH Phosphate Phosphorus Potassium Refractometry Relative Humidity Resistivity Salinity Silica Silver Sodium salt Sulphate Sulphide Sulphite Sulphur Dioxide Tartaric Acid Temperature Titration Turbidity Zinc.
You can use this chart to predict whether or not an atom can bond with another atomThe charge on an atom is related to its valence electrons or oxidation stateAn atom of an element is most stable when its outer electron shell is completely filled or half-filled. The positive ion cation is written first in the name. The first group is the three macronutrients that plants can obtain from water air or both carbon C hydrogen H and oxygen O.
Sulfur is a component of many proteins. 30 Count Pack of 1 48 out of 5 stars 876. 98 073Count Get it as soon as Thu Dec 2.
Benefits The high levels of nutrients in turnip greens can enhance health and. FREE Shipping on orders over 25 shipped by Amazon. Probiotic Vitamin C Vitamin D and Zinc Elderberry Immune Support for Kids Mixed Berry Chewables 30 Count.
Turnip greens also contain more than 250 mg of nitrate levels for every 100 grams of leaf which is a very high level. Jicama is packed with nutrients and may provide various health benefits including improved digestion weight loss and a reduced risk of disease. Test your Knowledge on Chemical formula.
Each element involved in these nutrients provides a different benefit. 2017 as well as offer relief for Wilson. AMMONIUM NITRATE POTASSIUM CHLORATE POTASSIUM NITRATE.
Of water or crushed dolomitic limestone to the soil can help address magnesium deficiencies. Put your understanding of this concept. This is a chart of the most common charges for atoms of the chemical elements.
Sodium thiosulfate ____Na2S2O3_____ 3. Formula and data book Chemistry v13.
Cobalt Iii Nitrate Con3o9 Chemspider
Copy this to my account.
Cobalt iii nitrate formula. 83762 free acid basis Compare Product No. K 4FeCN 6 Answer. You should complete this by Sunday.
Aluminum and sulfur. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. The negative ion anion is written second in the name.
Co2SO43 cobalt III sulfate 60. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Ionic Compounds Naming and Formula Writing.
13 potassium fluoride is KF 14 ammonium sulfate is NH 42SO 4 15 magnesium iodide is MgI 2 16 copper II sulfite is CuSO 3 17 aluminum phosphate is AlPO 4 18 lead II nitrite is PbNO 22. Chemical Formula Nomenclature Practice. Cobalt in the complex ion must be 3.
8 water molecules octahydrate. Cobalt III cobaltic Co 3 3 sodium Na 1 hydroxide OH 1 cobalt IV Co 4 4 tin II stannous Sn 2 2 iodate IO 3 1 copper I cuprous Cu 1 tin IV stannic Sn 4 4 iodide I 1 copper II cupric Cu 2 2 titanium II Ti 2 2 nitrate NO 3 1 gold I aurous Au 1 titanium III Ti. 2 of 15 Physical constants and unit conversions Physical constants and unit conversions Absolute zero Atomic mass unit Avogadros constant Ideal gas constant Ionic product constant for water at 298.
Polyatomic ions are ions which consist of more than one atom. Ionic Compounds Naming and Formula Writing. Formula 21 sodium phosphide Na 3 P 22 magnesium nitrate MgNO 3 2 23 lead II sulfite PbSO 3 24 calcium phosphate Ca 3 PO 4 3 25 ammonium sulfate NH 4 2 SO 4 26 silver cyanide AgCN 27 aluminum sulfide Al 2 S 3 28 beryllium chloride BeCl 2 29 copper I arsenide Cu 3.
10 MnNO 33 is manganese III nitrate 11 FePO 4 is iron III phosphate 12 CoCO 3 is cobalt II carbonate Name to formula problems. Write a formula for the ionic compound that forms from each pair of elements. -ite -ito For neutral ligands the common name of the.
Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. E-mail to a friend. E-mail to a friend.
Chromium III Sulfate Formula. Write a formula for the ionic compound that forms from each pair of elements. The metal atom or ion is written before the ligands in the chemical formula.
Chemical Formula For Copper. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound. Again remember that you never have to indicate the number of cations and anions in the name of an ionic compound.
Manganese II carbonate MnCO3 73. Cobalt II Sulfate Formula. The names of some common ligands are listed in Table 1.
Compound Name Type of Compound. Write the chemical formula for each of the following compounds. Formula writing rules to write the correct chemical formulas for each compound.
Potassium carbonate K2CO3 22. TrisethylenediaminecobaltIII chloride is an inorganic compound with the formula Coen 3Cl 3 where en is the abbreviation for ethylenediamineIt is the chloride salt of the coordination complex Coen 3 3This trication was important in the history of coordination chemistry because of its stability and its stereochemistryMany different salts have been described. It was designed by Alfred Stock 1876-1946 a German chemist and first published in 1919.
Sulfate nitrate and -ite eg. Give the formula for the following. Sulfur dioxide ____SO2_____ 2.
Iron III phosphate 66 K 3 N potassium nitride 67 SO 2 sulfur dioxide 68 CuOH copper I hydroxide 69 ZnNO 2 2 zinc nitrite 70 V 2 S 3 vanadium III sulfide Write the formulas for the following chemical compounds. Nirite change the endings as follows. Sodium peroxide Na2O2 63.
3Co cobaltIII ion cobaltic ion Copper Cu copperI ion cuprous ion 2Cu copperII ion cupric ion Gold Au3 goldIII ion Iron Fe2 ironII ion ferrous ion 3Fe ironIII ion ferric ion Manganese Mn2 manganeseII ion manganous ion 3Mn manganeseIII ion manganic ion Mercury Hg 2 2 mercuryI ion mercurous ion 2Hg mercuryII ion mercuric ion Nickel 2Ni nickelII ion Silver Ag. Potassium is the cation and the complex ion is the anion. Sodium nitrite NaNO2 31.
Write a formula for the ionic compound that forms from each pair of elements. Copper II Carbonate Formula. 1 of 15 Formulas Processing of data Chemical reactions reactants products and energy change Aqueous solutions and acidity Chemical equilibrium systems.
His study of chemistry began in Karlsruhe Germany. Greek prefixes are attached to the word hydrate to indicate the number of water molecules per formula unit for the compound eg BaOH 2 8H 2 O. Use the stock form for the transition metals.
Copy this to my account. Copper I Chloride Formula. The names of some common ligands are listed in Table 1.
Complete these in lab and on your own time for practice. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Empirical Formula Hill Notation.
Sodium and sulfur Express your answer as a chemical formula. Combined gas law formula. 71 silicon dioxide SiO 2 72 nickel III sulfide Ni.
For anionic ligands end in -o. When the chemical formula for a hydrated ionic compound is written the formula for the ionic compound is separated from the waters of hydration by a centered dot. HexaamminecobaltIII chloride is the chemical compound with the formula CoNH 3 6Cl 3It is the chloride salt of the coordination complex CoNH 3 6 3 which is considered an archetypal Werner complex named after the pioneer of coordination chemistry Alfred WernerThe cation itself is a metal ammine complex with six ammonia ligands attached to the cobaltIII ion.
Name Formula Charge Name Formula Charge Name Formula Charge. Potassium bromide KBr 62. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central.
C 25 H 42 N 7 O 17 P 3 S xLi CAS No. The positive ion cation is written first in the name. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atoms.
Barium sulfide BaS 72. Chromium VI Oxide Formula. For anions that end in -ideeg.
Cobalt II Nitrate Formula. Strontium and oxygen Express your answer as a chemical formula. Since there are 4 K.
History- The type of naming you will learn about is called the Stock system or Stocks system. Formula Moles of AgCl precipitated per mole of the compounds with excess AgNO3 i PdCl24NH3 2 ii NiCl26H2O 2 iii PtCl42HCl 0 iv CoCl34NH3 1 v PtCl22NH3 0 Example 91 WernerWernerWerner was born on December 12 1866 in Mülhouse a small community in the French province of Alsace. Chromium III Nitrate Formula.
The atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit. KMnO4 potassium permanganate Write the chemical formula for each of the following ionic compounds.
It is used as a catalyst in. 13 They exhibit a slow setting time 6 shrinkage on setting solubility 158 235 and they can stain tooth structure.

The Biological Inorganic Chemistry Of Zinc Ions Sciencedirect
Noncombustible but accelerates the burning of combustible materials.
Zinc nitrate solubility. Toxic oxides of nitrogen are produced in fires involving this material. Compare the rate of reaction between zinc and sulfuric acid with copper as a catalyst in this simple class practical. Even the nitrate products are oxidizing agents.
Prolonged exposure to fire or heat may result in an explosion. 1 Nickel IIIchloride potassium phosphate -- Molecular equation. The reaction between metallic zinc and hydrogen chloride gas yields the anhydrous form of zinc chloride.
Catalysts for the thermal decomposition of potassium chlorate. Method II Ion. The mean plasma zinc area under the curve for zinc.
It is soluble in both water and alcohol. K sp M n m A m- n. Zinc nitrate is an inorganic chemical compound with the formula ZnNO 3 2.
Zinc zn2 lead ii pb2 aluminum al3 fluoride f- soluble slightly soluble soluble soluble insoluble insoluble slightly soluble slightly soluble slightly soluble soluble soluble soluble insoluble slightly soluble chloride cl- soluble soluble soluble soluble soluble soluble soluble soluble soluble soluble insoluble soluble insoluble soluble bromide br- soluble soluble soluble. However the microemulsion was prepared from ZnNO 3 2 cyclohexane acrylonitrile-butadiene-styrene copolymer ABS butanol and hydrogen peroxide H 2 O 2. Whether or not such a reaction occurs can be determined by using the solubility rules.
All processes were carried out in a reactor. An early zinc. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website.
Zinc hydroxide Zn 2 is an inorganic chemical compoundIt also occurs naturally as 3 rare minerals. Magnesium nitrate is a crystalline source with high water solubility for use consistent with nitrates and lower acidic pH. Like the hydroxides of other metals such as lead aluminium beryllium tin and chromium Zinc hydroxide and Zinc oxide is amphotericThus it will dissolve readily in a dilute solution of a strong acid such as HCl and.
If large quantities are involved in a fire or the combustible material is finely divided an explosion may result. It is important to note that the zinc-chlorine bond in ZnCl 2 possesses some covalent characteristics which accounts for its low melting point and its solubility in ethereal solvents. Metallic zinc is an ideal anode material for aqueous batteries but suffers from irreversibility issues.
Beginning with Volume 1 covering the solubility of helium and neon in 1979 work has continued dealing with a wide array of solubilities of gases liquids and solids in binary. Method I Thorium Nitrate Colorimetric Method. State the method you would choose to prepare the following salts.
After fertilization crops take up a relatively small part of added nitrogen compounds namely 25-30. In association with Nuffield Foundation. The most widely applied nitrogen fertilizers are probably NaNO 3 sodium nitrate and NH 4 NO 3 ammonium nitrate.
Wülfingite orthorhombic ashoverite and sweetite both tetragonal. For ionic compounds with limited solubility in water an equilibrium constant K sp can be defined from the ion concentration in water from the equation. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution. Cd 3 AsO 4 2. 75 320 An advantage to this sealer group is antimicrobial activity.
A solidsolid reaction between lead nitrate and potassium iodide. A sodium carbonate b calcium chloride c barium sulphate d leadII nitrate e leadII chloride. The residue ends up in groundwater and surface water through soils because nitrates are water soluble.
Under either high pH 5 or low pH 3 intragastric pH conditions. Where M m A n is the slightly soluble substance and M n and A m-are the ions produced in solution by dissosiation of M m A n. Melting point - the temperature at which a solid turns into a liquid.
Zinc oxideeugenol sealers will absorb if extruded into the periradicular tissues. Organic fertilizers mainly contain nitrogen as proteins urea or. This is our newest publication and has been created to support the school technician profession in Scotland.
The solubility of zinc salts is affected by gastric pH. Nitrate compounds are usually soluble in water. Back to top Citing this page.
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. In the mid-1970s a group of chemists and chemical engineers came together within IUPAC to exhaustively compile and critically evaluate reports of experimental measurements of solubility primary chemical literature. The nitrate compounds can form a flammable mixture when combined with hydrocarbons.
The chemical equation for. An aqueous electrolyte based on Zn and lithium salts using either LiMn2O4 or O2 cathodes now. Healthy subjects were given a single oral dose of 50 mg elemental zinc as the acetate.
Zinc salts are not equal in solubility which is important in zinc absorption. Organic Ions and Compounds. List of 131 compounds in the Solution Calculator database for which the density function is defined are for the Perrys Chemical Engineers Handbook.
Try this demonstration to investigate the effectiveness of. Zinc oxideeugenol sealers have a history of successful use over an extended period of time. Use solubility rulesactivity tables and tables for strong bases and acids to write the equations.
Boiling point - the temperature at which a liquid turns into a gas. The emulsion consisted of ZnNO 3 2 and an appropriate surfactant cationic anionic or non-ionic. Zinc nitrate is a colorless crystalline solid.
Includes kit list and safety instructions. This white crystalline salt is highly deliquescent and is typically encountered as a hexahydrate ZnNO 3 2 6H 2 O. In all of these methods the starting solution used was zinc nitrate ZnNO 3 2.
One way of controlling cadmium in effluent streams is to add sodium hydroxide which precipitates insoluble. A potassium nitrate b zinc nitrate c magnesium sulphate d copperII carbonate. Ksp solubility product constants of many popular salts at SolubilityOFthings.
Cadmium is a highly toxic environmental pollutant that enters wastewaters associated with zinc smelting Cd and Zn commonly occur together in ZnS ores and in some electroplating processes. Perry Perrys Chemical Engineers Handbook McGraw-Hill 2 99-118 2008. M m A n s mM n aq nA m-aq.
State whether the following salts are soluble or insoluble. The table below gives calculated values of K.
And the more we experiment with it the more we uncover various truths about it. Water has a high specific heat.
The color change in phenolphthalein is a result of ionization and this alters the shape of the phenolphthalein molecules.

Zinc nitrate physical properties. 2017 as well as offer relief for Wilsons disease a genetic disorder whereby the body stores toxic levels of copper. Nitrogen is an essential element required for successful plant growth. Other applications are in dentistry and in high-capacity zinc long-life batteries.
The rosin increases fracture resistance and the zinc acetate is effective in accelerating the reaction rate. The liquid is a preparation of eugenol which reacts with the powder to form an amorphous chelate of zinc eugenolate. He then made a solution of didymium nitrate and added ammonium hydroxide.
Toxic oxides of nitrogen are produced in fires involving this material. It is also soluble in acetone ethanol and glycerol. 212 contains zinc oxide rosin and zinc acetate in the powder.
The four polymorphs of ZnCl 2 feature a tetrahedral coordinate geometry between the Zn 2 ions and the Cl Molten zinc chloride is. Magnesium nitrate MgNO32 - Magnesium nitrate is the chemical name of MgNO32. Zinc nitrate is a colorless crystalline solid.
If large quantities are involved in a fire or the combustible material is finely divided an explosion may result. Visit BYJUS to understand the properties structure and uses of Magnesium nitrate. In materials science zinc oxide is classified as a semiconductor in group II-VI whose covalence is on the boundary between ionic and covalent.
Its in the food we eat and the air we breathe. By the way if its not actually the holidays and I havent updated this recently sorry about that. Zinc acetate is another chemically-altered form of zinc and considered to be more absorbable than gluconate.
Silvers catalytic properties make it ideal for use as a catalyst in oxidation reactions. The synthesis is typically carried out at temperatures of about 90 C in an equimolar aqueous solution of zinc nitrate and hexamine the latter providing the basic environment. Silver levels in soil are not usually high except in mineral-rich areas when they can sometimes be as much as 44 ppm.
Although inorganic nitrogen compounds ie NH 4 NO 2 and NO 3 account for less than 5 of the total nitrogen in soil they are the main form of the element absorbed by most plantsInorganic and organic fertilizers are applied to maintain the nutritional condition of different cropping systems. That is both solute and solvent can be recovered in chemically unchanged forms using appropriate separation methods. This form may aid in reducing the duration of the common cold JRSM Open.
This classification relates to the dependency of the properties upon the size or extent of the system or object in question. Specific heat is the amount of energy required to change the temperature of a substance. In alkaline it turns pink.
Physical properties of materials and systems are often described as intensive and extensive properties. Plants can absorb silver and. The longer involves a recap of the properties of metals.
Zinc Acetate is made from zinc nitrate and acetic anhydride. Zinc chloride is solid at room temperature and has a white crystalline appearance. He concentrated his attention on the first precipitate and measured its spectrum which revealed it to be a new element samarium.
Zinc acetate is the best. The toxicity of silver was significantly less in the zinc-pretreated cells. Zinc is a bluish-white lustrous diamagnetic metal though.
Samarium itself was eventually to yield other rare-earths. Potassium nitrate sulfur and carbon Gunpowder Oxygen and water Sea foam. Certain additives such as polyethylene glycol or polyethylenimine can improve the aspect ratio of the ZnO nanowires.
Isnt it interesting to consider how science is all around us. Black powder is. Water has several other unique physical properties.
I promise that I wont take your. Im glad you found your way here for chemistry help because youre in the right spot to be enlightened. For example solid zinc nitrate dissolves in water to form an aqueous solution of zinc nitrate.
Gadolinium in 1886 and europium in 1901. Here are the rules for using this free website. Zinc aluminum magnesium and copper Zamak Advertisement Science Is All Around Us.
To be suitable for use water must be free from all impurities that are offensive to the sense of sight taste or smell and one very important physical characteristic that should be encountered when discussing water quality is turbidity Davis and Cornwell 2012. Its a blend of potassium nitrate saltpeter charcoal and sulfur in a 751510 ratio. A zincblende unit cell.
The nitrate ZnNO 3 2 chlorate ZnClO 3 2 sulfate ZnSO 4 phosphate Zn 3 PO 4 2 molybdate ZnMoO 4 cyanide ZnCN 2 arsenite ZnAsO 2 2 arsenate ZnAsO 4 2 8H 2 O and the chromate ZnCrO 4 one of the few colored zinc compounds are a few examples of other common inorganic compounds of zinc. Your Post-apocalypse Chemistry Connection. As a result pure.
An intensive property is a bulk property meaning that it is a physical property of a system that does. Prolonged exposure to fire or heat may result in an explosion. In acidic solutions it is colorless.
It is used as a catalyst in. The phenolphthalein indicator allows chemists to visually identify whether a substance is an acid or a base. Physical properties of water are related to the appearance of water namely the color temperature turbidity taste and odor.
Noncombustible but accelerates the burning of combustible materials. The formation of a solution from a solute and a solvent is a physical process not a chemical one. Physical properties are used to observe and describe matter.
Because water has a high specific heat it can absorb large amounts of heat energy before it begins to get hot. He observed that the precipitate which formed came down in two stages. Zinc oxide with its unique physical and chemical properties such as high chemical stability high electrochemical coupling coefficient broad range of radiation absorption and high photostability is a multifunctional material 12.
The zinc oxideeugenol cements are used to provide a sedative. Doping of the. The solubility of this compound in water corresponds to 432g100g.
Water in a pure state has a neutral pH. Following pretreatment with zinc chloride hepatocytes were treated with 20 or 40 uM silver nitrate for 24 hr and cytotoxicity was then assessed by enzyme leakage and loss of intracellular potassium. Zinc oxideeugenol cement Fig.
The best way to learn new. Physical Science Text. One of the key ingredients for firecrackers ground fireworks and aerial fireworks ones which explode in the sky is black powder invented by the Chinese about 1000 years ago.
Silver in the environment. Physical Properties of Zinc chloride.