Its structure is given below Image will be uploaded soon. 11545 K anhydrous 122.
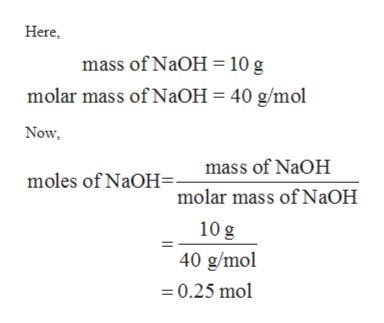
Answered What Is The Molarity M Of A N 10 Bartleby
Potassium carbonate K 2 CO 3 2.
Sodium hydroxide formula and molar mass. Substitute the known values to calculate the molarity. 1528 gcm 3 20 C anhydrous 145 gcm 3 20 C trihydrate Melting point. To learn about the structure Properties Preparation Uses Health Hazards and FAQs of Sodium hydroxide NaOH.
Sodium sulfate Na 2 SO 4. Make sure to. Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound.
Sodium hypochlorite pentahydrate is a greenish yellow solid. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Sodium hydroxide is also the most common base used in chemical laboratories.
You can convert between wv mv or weight ratio percentage concentration and molarity mol L-1 using the molar mass of the solute. CuCl 2 CuCl2 C 12 H 22 O 11 C12H22O11 C 6 H 5 3 PCCO C18H15PCCO Formula. Solubility Table Table of Solubilities Water is a commonly used solvent so it is very useful to construct a table of solubilities based on the mass of a solute that will dissolve in a given volume of water.
Vinegar acetic acid odor when heated to decomposition. An alkali caustic soda is widely used in many industries mostly as a strong chemical base in the manufacture of pulp and paper textiles drinking water and detergents. 597 K anhydrous 58 C 136 F.
Exhaust from a chimney contains 10 mol of oxygen O 2 53 mol of nitrogen N 2 and 37 mol of carbon dioxide CO 2. Convert the expressions above to obtain a molarity formula. Soda caustic CAS.
Properties of Sodium Hypochlorite. The molar concentration of sulphuric acid is 400 mol L 1. Sodium hydroxide MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents.
Calculate the mass of sodium sulfate made when 20 g of sodium hydroxide reacts with excess sulfuric acid. It is also commonly known as caustic soda or Iye and is widely used in manufacturing a variety of products such as paper soap and detergents pulp explosives liquid drain and oven cleaners etc. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH.
The tool is designed so you can flip between different parts of a problem set. The molar mass of sodium hydroxide is 40 g mol 1. 82034 gmol 1 Appearance White deliquescent powder Odor.
324 C 615 F. B the chemical formula of the compound appears after the arrow. Worldwide production in 1998 was around 45 million tonnes.
The solute is the. It is also known as the sodium salt of hypochlorous acid HOCl. What is the mass of 356 x 1025 formula units of copper II sulfate.
We recommend you bookmark it so you can refer back to it. Here the sodium hydroxide solution is not a primary standard and is taken in a burette and a known volume of the oxalic acid a standard solution is taken in the titration flask. Worksheet Mole Conversions Name.
The molar mass of sodium hydroxide is 40 g mol 1. The molar mass of oxygen nitrogen and. Covalent compounds are composed of non-metallic elements.
As mass volume molarity molar mass then mass volume molar mass molarity. The chemical formula calculator also contains the names of a range of covalent compounds which occur as acids. Uses the formula of a reactant to determine molar mass.
Sodium hypochlorite shows following physical and chemical properties Its molar mass is 744 gmol-1. Molar Mass of Frequently Calculated Chemicals. The density of the solution is 102 g cm 3.
The A r of sodium is 23 and the A r of oxygen is 16. Find the mass concentration of H 2 SO 4 and SO 4. The molar mass of H 2 SO 4 and SO 4 is 981 g mol 1 and 961 g mol 1.
Enter formulas with proper capitalization and unpack brackets. What is the number of molecules present in 5000 g of diphosphorus pentoxide. The rates of hydrolysis by sodium hydroxide in 875 weight per cent aqueous ethanol have been measured at 0 and 30 of ethyl phenoxyacetate and the 2- and 4-fluoro chloro bromo and iodo and.
What is the mass of 125 x 1023 sodium ions. Molar Mass of Sodium carbonate Na2CO3 Molar Mass of Sodium nitrate NaNO3 Molar Mass of Sulfurous acid H2SO3 Molar Mass of Zinc sulfate ZnSO4 Bookmarking Save and Share Results. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily.
Show all work utilizing dimensional analysis wherever possible. Aluminium hydroxide AlOH 3 is used as an antacida chemical used to. A r of H 1 A r of O 16 A r of Na 23 A r of S 32 Reveal answer.
Determine the molar mass of sodium oxide Na 2 O. Sodium Hydroxide is one of the simplest hydroxides. The molarity of a sodium hydroxide solution is 051 M.
Molarity 5 12 3646 0114 moll 0114 M. Simply type in the remaining values and watch it do. 106462 View Pricing Availability.
Download Product Safety Card. Water is the solvent. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
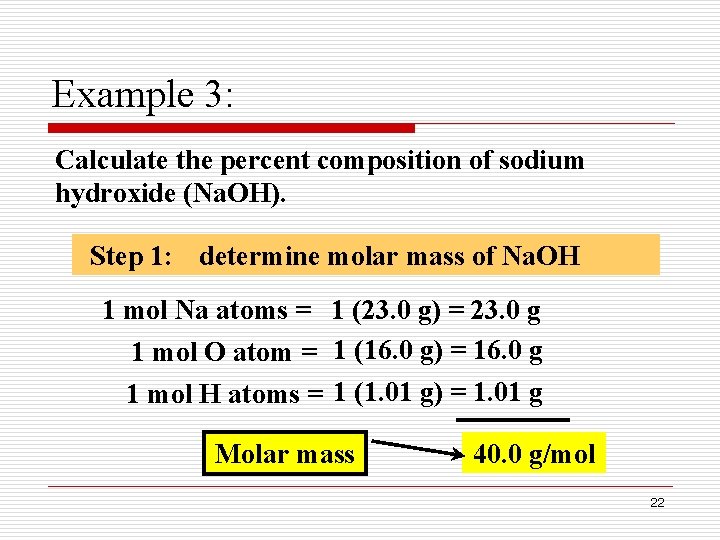
The M r of sodium oxide is 23 2 16 62. 40 gmol Chemical Formula. Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base.
C 2 H 3 Na O 2. 8814 C 16185 F. Due to such characteristics it is often used with neutral water and acidic HCl acid to.
Determine the molar mass of calcium sulfide CaS. It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps. The molecular weight of sodium hydroxide is 40 gmol.
You can also use this molarity calculator to find the mass concentration or molar mass. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Include all units and account for significant figures.
What is the molar mass of. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Determine the molar mass of ozone.
Sodium Hydroxide chemical formula is written as NaOH. 331 K trihydrate Boiling point. Visit BYJUS for more information.
To determine the strength of the sodium hydroxide solution by titrating it against a standard solution of oxalic acid. The relative formula mass of a substance shown in grams is called one mole of that.

Molar Mass Molecular Weight Of Naoh Sodium Hydroxide Youtube

Quiz Worksheet Molar Mass Study Com

Sodium Hydroxide Caustic Soda Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs Lewis Structure Infographic
Sodium Hydroxide Hnao Chemspider

Answered What Mass Of Sodium Hydroxide Naoh Bartleby
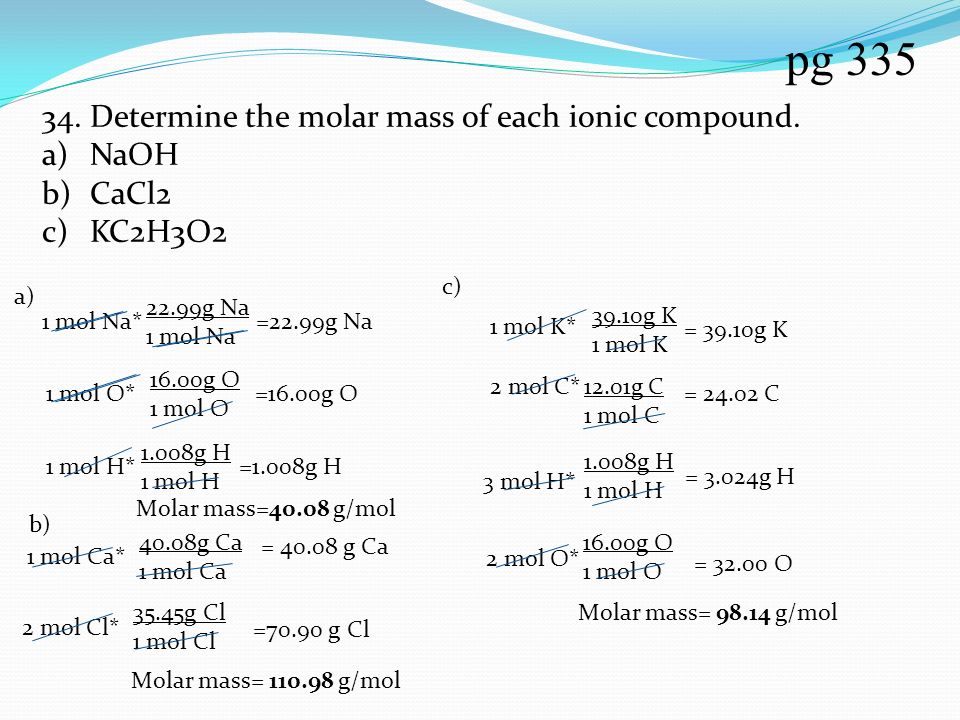
Pg Determine The Molar Mass Of Each Ionic Compound Naoh Cacl2 Ppt Download

Molar Mass Molecular Weight Of Naoh Sodium Hydroxide Youtube

Chapter 6 Chemical Composition 2006 Prentice Hall Chapter