This difference becomes important at very low temperatures since orthohydrogen becomes unstable and changes to the more stable parahydrogen arrangement releasing heat in the process. For a new antibody we recommend starting with three sides.

Scientists Turn Methane Into Methanol At Room Temperature
Vapor definition a visible exhalation as fog mist steam smoke or noxious gas diffused through or suspended in the air.

Methanol state at room temperature. Methanol is an industrial organic solvent commonly used in organic synthesis. Preserve approximately 25 grams of soilwith 25 ml of methanol in a tared 60-ml vial. Subsequently samples were incubated with primary antibodies against α-actinin 1500.
Over 75 of normal hydrogen at room temperature is ortho-hydrogen. Low temperature systems can reduce costs by reducing insulation materials start-up and degradation-related costs. The high reaction temperature and unremovable oleylamine result in a tough synthetic process.
Samples prepared in 5050 Water. Aim for an overall healthful dietary pattern the American Heart Association advises rather than focusing on good or bad foods. Methanol is highly toxic if inhaled or ingested and may even lead to.
These data outperform methanol productivities from methane oxidation over the state-of-the-art AuPd catalysts reported under similar reaction conditions in the literature table S4 including the AuPdTiO 2 catalyst mixed organic reductant and O 2 methanol productivity. After back flushing or washing at 50 deg celsius using 6040. With higher operating temperatures the temperature gradient increases the.
Moreover the much higher price of acetylacetone platinum and ruthenium acetylacetone than chloroplatinic acid and ruthenium chloride increases cost. Other sample sizes such as. Because the alkali metal.
It encourages learners to develop confidence in and a positive attitude towards chemistry and to recognise its importance in their own lives and to society. The Solid State Energy Conversion Alliance 2008 interim target for overall degradation per 1000 hours is 40. Research is going now in the direction of lower-temperature SOFCs 600 C.
Carbon is one of the most important elements to life on planet Earth. 40 μmol Ru 3 CO 12 and 40 μmol Rh 2 OAc 4 based on metals 075 mmol imidazole 3 mmol LiI 2 ml DMI 12 mmol MeOH 4 MPa CO 2. Lithium melts at 181ºC sodium at 98ºC potassium at 63ºC rubidium at 39ºC and cesium at 28ºC.
It is being considered as a potential hydrogen source in fuel cell technology due to its high HC ratio low propensity for soot generation relatively low reforming temperature and its liquid state at room temperature. Carbon has been known about since ancient times. There is no notable change of the Cu state after the reduction of CuMo 2 CT x SiO 205 h exposed to air and then treated under H 2 from room temperature to 500 C for 55 h at 500 C.
Abcam at 4 C overnight. To develop a method to prepare PtRu nanodendrites using cheaper Pt and Ru precursors in aqueous and at room temperature is. Methanol - Thermophysical Properties - Chemical physical and thermal properties of methanol CH 3 OH also called carbinol wood alcohol hydroxy methyl and methyl alcohol.
The vapors rising from the bogs. Is an exothermic reaction at room temperature ΔH298 K - 118 kcalmol but it competes. When required leave to warm at room temperature for 5 min.
Sigma-Aldrich and cTnT 1500. A dimethyl ether 45 amu x33 b hydrogen 2 amu x1 c CO 2 44 amu x33 d methanol 29 amu e formaldehyde 31 amu x10 f CO 28 amu x1. 55 A carbon dioxide concentration.
55 The method has been applied to numerous polymers. Sodium and potassium can be cut with a butter knife. 105 mmol g AuPd 1 hour 1 and AuPd.
Our ASA level Chemistry specification provides a broad coherent satisfying and worthwhile course of study. A solid state nanofoaming method about or less than 100 nm has been developed by saturating thermoplastic polymers with liquid carbon dioxide at low saturation temperatures of below room temperature. After 30-50 injections the pressure rises to 170-200 bars initial pressure 96-106 bars.
A polar solvent methanol acquired the name wood alcohol because. The alkali metals are solids at room temperature except for hydrogen but have fairly low melting points. Composition of Other Fuels It is natural for.
Briefly cells were fixed for 10 min with 4 paraformaldehyde permeabilized in 025 Triton X-100 Sigma-Aldrich and 05 M ammonium chloride in 025 M TBS pH 74 and blocked with 08 bovine serum albumin BSA for 1 h at room temperature. Personal Health A Heart-Healthy Way to Eat. Desorption products after exposure of a CuZnOAl 2 O 3 catalyst to methanol at ambient temperature.
The olefins ethylene or propylene formed from methanol via MTO methanol-to-olefins process. Methanol exists as a clear and colourless volatile liquid at room temperature The density of Methanol is 792 kgm 3 The melting point of Methanol is -976 C Its odour is very similar to Ethanol which is used as drinking alcohol and is often mistaken for Ethanol and results in alcohol poisoning. Methanol in the vapor state_ The pressure is saturated -apor pressure at room temperature except lor the upper curve which is at a pressure 01 6 em 01 Hg.
As a result of all this work the mechanism of the reaction was concluded to be the following Equations 3 and 3 3 That is CO 2 is directly. This heat can complicate low temperature hydrogen processes particularly liquefaction. Figure 1 shows an Arrhenius plot constructed with steady-state rates for the synthesis of methanol and the RWGS reaction on Cu111 and on a ZnO000 surface with a Cu coverage of 02 ML of Cu47 At this Cu coverage the results of scanning tunneling microscopy STM suggest that two- and three.
Although this resulted in some loss in the total re flected energy the stray radiation was reduced considerably. They are also relatively soft metals. 18 to 21 diamond.
Methanol also known as methyl alcohol amongst other names is a chemical and the simplest alcohol with the formula C H 3 O H a methyl group linked to a hydroxyl group often abbreviated MeOH. Phase at Room Temperature. When methanol is used as the preservative GRO and VOC results can be obtained from the same sample.
Prepare fixative acetone methanol or ethanol at room temperature. 55 After saturation material is heated to above the glass transition temperature of the polymer. 1 Paraformaldehyde 2 Acetone 3 11 solution of acetonealcohol methanol or ethanol Fix with the fixative for 15 min at room temperature.
A maximum of 35 grams of soil is recommended to enable a 11 ratio of soil to extraction solvent in the sample container. 359 mmol g AuPd 1 hour 1 or mixed H 2 and O 2 methanol productivity. Salts of the Group 1A elements tend to be extremely soluble in water.
Mixing of Humid Air - The change in state wwhen mixing moist air -. It is a light volatile colourless flammable liquid with a distinctive alcoholic odour similar to that of ethanol potable alcohol. 2267 grams per cm cubed.

Methanol Dynamic And Kinematic Viscosity Vs Temperature And Pressure
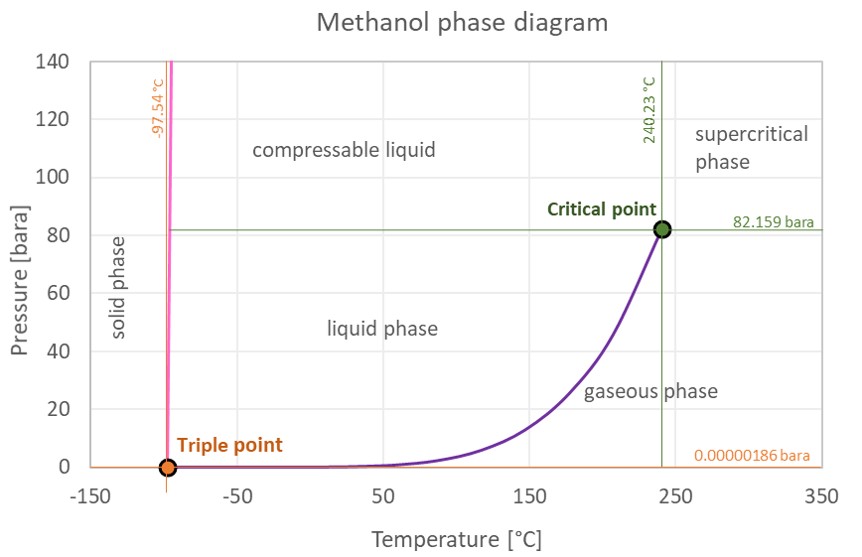
Methanol Thermophysical Properties
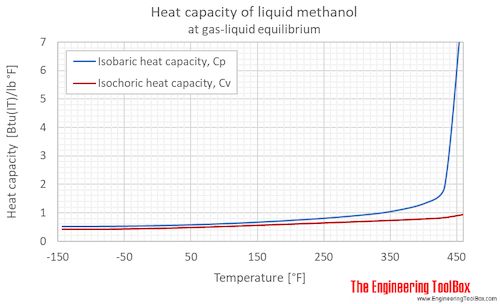
Methanol Specific Heat Vs Temperature And Pressure
Vapor Pressure Of Methanol From Dortmund Data Bank
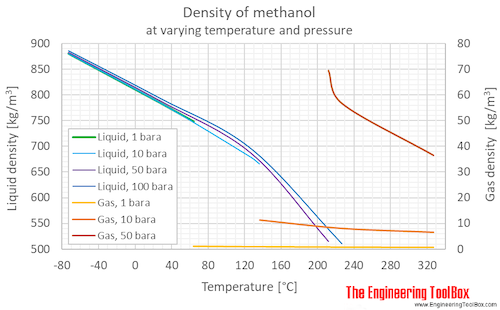
Methanol Density And Specific Weight Vs Temperature And Pressure
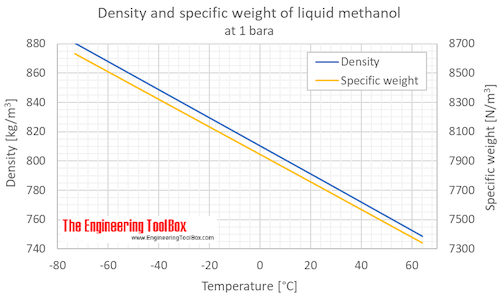
Methanol Density And Specific Weight Vs Temperature And Pressure

