Elementary lead does not dissolve in water under normal conditions 20 o C and pressure 1 bar. Ionic Compound Formula K sp.

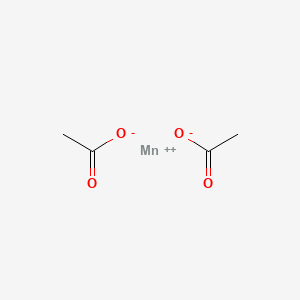
Manganese Ii Acetate Wikipedia
Bacteria live on the skin in the mouth and in the nose but the great majority live in the gut primarily the large.

Manganese acetate solubility. The treatment and conditioning of boiler feed water must satisfy three main objectives. LeadII acetate potassium hydroxide PbOH2 sodium chromate nickelII iodide NiCrO4 strontium perchlorate ironII sulfate SrSO4 ammonium acetate cadmium nitrate none thalliumI nitrate calcium chloride TlCl sodium sulfide ironIIIchloride Fe2S3 NiCrO4 SrSO4 none PbOH2 TlCl Fe2S3 13035035 points Previous. Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution.
SOLUBILITY PRODUCT CONSTANTS The solubility product constant K sp is a useful parameter for calculating the aqueous solubility of sparingly soluble compounds under various conditions. Evaporation Rate Butyl Acetate 1 Not applicable for an Article. External treatment is the reduction or removal of impurities from water outside the boiler.
Not applicable for an Article. Rofarma Italy has a popular commercial starch acetate product AMPRAC01 marketed as a water-based polymer for controlled drug delivery of solid dosage form. At a temperature of 0 degrees celsius the solubility of benzoic acid in water corresponds to 17 grams per litre.
Henrys law states that the solubility of a gas is directly proportional to the partial pressure of the gas. In general external treatment is used when the amount of one or more of the feed water impurities is too high to be tolerated by the. Manganese Dioxide 3 CAS 1313-13-9 5 mgm3 Ceiling as Mn 02 mgm.
The RNA was denatured concentration gradient. γ-Mn 2 O 3 can be produced by oxidation followed by dehydration of manganeseII hydroxide. Solubility Rules or Table 104 on page 231 of your textbook.
Hg 2 Br 2. Thus if K sp Mm An is the equilibrium. M m A n s mM n aq nA m-aq.
The bacteria that live in the human body outnumber the bodys cells 10 to 1. K sp M n m A m- n. C 3 H 6 O 2.
It is reported to have a wide range of additives and excipients for improving storage stability taste and odor masking pH-dependent solubility protection of sensitive actives from gastric fluid protection of gastric mucosa from. It may be determined by direct measure-ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states. We would like to show you a description here but the site wont allow us.
Solubility of lead and lead compounds. ManganeseII acetate are chemical compounds with the formula MnCH 3 CO 2 2 H 2 On where n 0 2 4. Because the solubility of ferric iron in water or in 240 mL of 50 mM HEPES pH 80 200 mM sodium acetate final concentration that had been previously deoxygenated by plasma is so low 10218 M cells must combat a massive bubbling with argon for several hours.
The table below gives calculated values of K. Where M m A n is the slightly soluble substance and M n and A m-are the ions produced in solution by dissosiation of M m A n. The addition of chromate ion to.
Production of high quality steam. Solubility in Water by weight Not applicable for an Article. However the solubility of this compound in water increases when the temperature is increased as is the case with most compounds.
ManganeseII sulfide pink MnS. ManganeseII sulfide green MnS. When heated to 100 degrees celsius the solubility of this compound in water increases to 5631 grams per litre.
Mn 3 O 4 2 CH 3 CO 2 H MnCH 3. Some of these compounds are used as a catalyst and as fertilizer. Solubility Product Constants near 25 C.
Although PbCl 2 is insoluble at room temperature its solubility is increased dramatically at higher temperatures. The distribution of manganese depends on its chemical form valence and solubility. ManganeseIII oxide is a chemical compound with the formula Mn 2 O 3.
A well-known example of a water soluble lead compound is lead sugar leadIIacetate which derived its name from its sweet nature. It may however occur dissolved in water as PbCO 3 or PbCO 3 2 2-. Not applicable for an Article.
To measure the solubility product of leadII sulfate PbSO 4 at 25C you construct a galvanic cell like the one shown in Figure PageIndex1 which contains a 10 M solution of a very soluble Pb 2 salt leadII acetate trihydrate in one compartment that is connected by a salt bridge to a 10 M solution of Na 2 SO 4 saturated with PbSO 4 in the other. Pb 2 also forms an insoluble white sulfate which dissolves in a solution containing acetate ion due to the formation of the weak electrolyte PbCH 3 COO 2. In a dietary study levels of manganese in the liver and kidney were significantly higher in male mice exposed to manganese acetate or manganese carbonate than in mice exposed to manganese chloride or manganese dioxide Komura and Sakamoto 1991.
You then insert a Pb electrode. Pressure can also affect solubility but only for gases that are in liquids. Temperature affects the solubility of both solids and gases but hasnt been found to have a defined impact on the solubility of liquids.
C 6 H 14 O 6. It dissolves readily in boiling water. These materials are white or pale pink solids.
ManganeseII acetate can be formed by treating either manganeseIIIII oxide or manganeseII carbonate with acetic acid. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3. MnC 2 O 4 x 2H 2 O.
The transition from benign and abundant and renatured using a thermal cycler by. Heating MnO 2 in air at below 800 C produces α-Mn 2 O 3 higher temperatures produce Mn 3 O 4. Ksp solubility product constants of many popular salts at SolubilityOFthings.
For ionic compounds with limited solubility in water an equilibrium constant K sp can be defined from the ion concentration in water from the equation. Boiling Point 760 mm Hg C Not applicable for an Article.
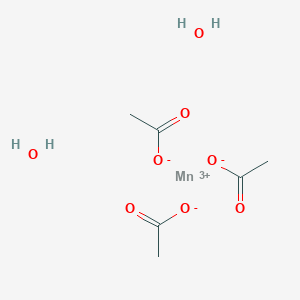
Manganese Iii Acetate Dihydrate American Elements
Manganese Ii Acetate C4h6mno4 Pubchem

Manganese Ii Acetate Wikipedia
Simultaneous Tg Dta Curve Of Manganese Acetate Measured In An Air Flow Download Scientific Diagram
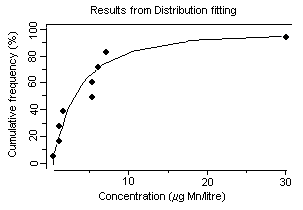
Manganese And Its Compounds Environmental Aspects Cicads 63 2004

Manganese Iii Acetate Mediated Synthesis Of Polysubstituted Pyrroles Under Solvent Free Ball Milling Sciencedirect
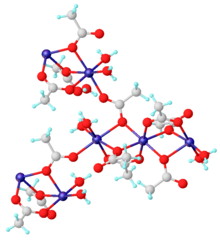
Manganese Ii Acetate Wikipedia
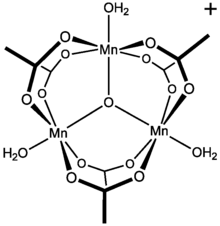
Manganese Iii Acetate Wikipedia

Diagram Of Manganese Species In Solution Vs Ph Activity Coefficient Download Scientific Diagram