There was no correlation between duodenal pH and duodenal soluble Cu concentrations P 098. The product is calcium hydrogen carbonate.
Chemistry Copper Carbonate Acitivity
For ionic compounds with limited solubility in water an equilibrium constant K sp can be defined from the ion concentration in water from the equation.

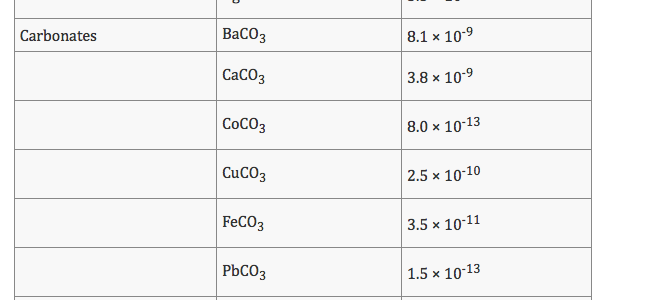
Copper carbonate solubility. Solubility Product Constants near 25 C. Doi10106313679678 IUPAC Project. K sp M n m A m- n.
It may be determined by direct measure-ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states. Calcium phosphate solubility is 20 mgL and that of calcium fluoride is 16 mgL. The following is for solubility in pure water not with any common ions.
Carbonate mineral any member of a family of minerals that contain the carbonate ion CO 3 2- as the basic structural and compositional unitThe carbonates are among the most widely distributed minerals in the Earths crust. For example in the process of. It is one of the simple methods to purify water.
Alters the release of neurotransmitters. The pH in the small intestine is an important factor in determining solubility of Cu as well as other minerals in the digesta Pang and Applegate 2007. The table below gives calculated values of K.
A tolerance of 1 ppm is established in potable water for residues of copper resulting from the use of the algicides or herbicides basic copper carbonate malachite copper sulfate copper monoethanolamine and copper triethanolamine to control aquatic plants in reservoirs lakes ponds irrigation ditches and other potential sources of potable water. Solubility in hydrochloric acid. Calcium carbonate has a solubility of 14 mgL which is multiplied by a factor five in presence of carbon dioxide.
M m A n s mM n aq nA m-aq. However jejunal pH and jejunal soluble Cu concentration were negatively correlated r 051. If compounds have different solubilities or relative solubilities they can be separated.
The table gives the solubility of potassium nitrate at six different temperatures. Jitka Eysseltováa and Roger Bouaziz Potassium Sulfate in Water JPCRD 2012 41 013103. Dirkse Copper Silver Gold and Zinc Cadmium Mercury Oxides and Hydroxides 1986.
AG105 AG203 and. 7110 9 x2x 2. Thus if K sp Mm An is the equilibrium.
Calcium carbonate 70 Salt 15 DL-Methionine. P block metal carbonate compounds. This compound is rarely encountered because it is difficult to prepare and readily reacts with water moisture from the air.
The chemicals are added to form particles which settle and remove contaminants from water. The solubility of silver carbonate is sensitive to the square of the metal-ion concentration because two silver ions per carbonate ion are necessary to build the solid crystal. Only high-silver content brazing alloys should be used for brazed joints at risk of corrosion eg.
Because of how the solubility constant is defined your answer will be in terms of moles of the compound dissolved per liter of water. A The percentage composition by mass of copper pyrites is Cu 3460 Fe 3052 S 3488 Show by calculation that the empirical formula of copper pyrites is CuFeS 2 3 b Copper is obtained from copper pyrites in a two-stage. Affects cyclic adenosine monophosphate concentrations.
CopperII carbonate or cupric carbonate is a chemical compound with formula CuCO 3. The terms copper carbonate copperII carbonate and. And blocks inositol.
Copper brazing alloys L-ZnCu42 formerly standardized in DIN 813 part 1 and CU301 EN 1044 and the silver solders AG306 and AG304 are preferred for the manganese-containing Cu-Ni alloys. Lithium Carbonate is the carbonate salt of lithium a soft alkali metal with antimanic and hematopoietic activities. Solubility and Related Thermodynamic Quantities of CadmiumII Carbonate in Aqueous Systems JPCRD 2011 40 043104.
Lead carbonate - PbCO 3. Calcium compounds are more or less water soluble. In this section we are going to discuss solubility and colours of p block metal carbonate compounds.
It is made by reacting copper with chlorine. At ambient temperatures it is an ionic solid a salt consisting of copperII cations Cu 2 and carbonate anions CO 2 3. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
These brazing alloys together with CU305 and AG203 are used for iron-containing alloys. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3. Boiling point - the temperature at which a liquid turns into a gas.
Therefore the form of the solubility product expression is different. SOLUBILITY PRODUCT CONSTANTS The solubility product constant K sp is a useful parameter for calculating the aqueous solubility of sparingly soluble compounds under various conditions. Solubility of calcium and calcium compounds Elementary calcium reacts with water.
Lithium interferes with transmembrane sodium exchange in nerve cells by affecting sodium potassium-stimulated adenosine triphosphatase Na K-ATPase. Solve for x and youll know how soluble the compound is. 7 Copper pyrites is an ore of copper that contains copper iron and sulfur.
It can also be made by reacting copperII hydroxide copperII oxide or copperII carbonate with hydrochloric acid and from pure copper and from 11 solution of hydrogen peroxide and hydrochloric acid where copper first get oxidized to CuO from H2O2 and then reacts with HCl to form CuCl2 reaction goes like this. Ionic Compound Formula K sp. The solubility rules the technique ie when the product of ion concentrations in simple in the solution over the solubility product of the respective solid the precipitation occurs.
Basic copper carbonate is a chemical compound more properly called copperII carbonate hydroxideIt is an ionic compound a salt consisting of the ions copperII Cu 2 carbonate CO 2 3 and hydroxide OH. Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution. Melting point - the temperature at which a solid turns into a liquid.
The name most commonly refers to the compound with formula Cu 2 CO 3 OH 2It is a green crystalline solid that occurs in nature as the mineral malachite. Aluminium carbonate is not stable in the water and hydrolysis to aluminium hydroxide white precipitate in the water. Aluminium carbonate - Al 2 CO 3 2.
You may need a calculator to find the final answer. The positive identification of carbonate minerals is aided greatly by the fact that the carbon-oxygen bond of the CO 3 group in carbonates becomes unstable and breaks down in the presence of hydrogen ions H available in acidsThis is expressed by the reaction 2H CO 2 3 H 2 O CO 2 which is the basis for the so-called fizz test with dilute. The treated water is reused whereas the settled portion is.
The crystal structure of many carbonate minerals reflects the trigonal symmetry of the carbonate ion which is composed of a carbon atom centrally located in an. Where M m A n is the slightly soluble substance and M n and A m-are the ions produced in solution by dissosiation of M m A n. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.

Soluble Or Insoluble General Solubility Guidelines Many Factors Affect Solubility So Predicting Solubility Is Neither Straightforward Nor Simple The Ppt Download

Solutions Aim To Investigate Solubility Of Some Compounds Method Water Copper Ii Carbonate Water Copper Ii Chloride Water Potassium Chloride Ppt Download

Is Cuco3 Soluble Or Insoluble In Water Youtube
Solved The Value Of The Solubility Product Constant For Chegg Com
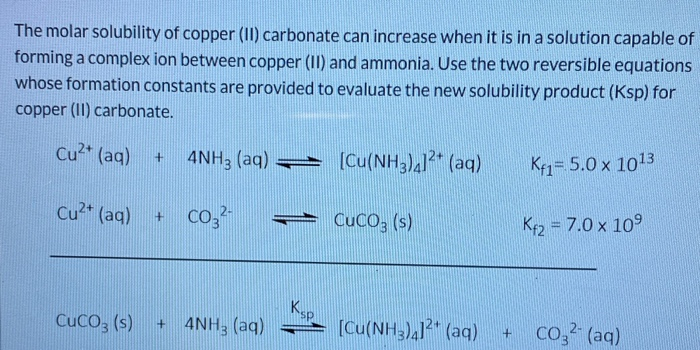
Solved The Molar Solubility Of Copper Ii Carbonate Can Chegg Com

Basic Copper Carbonate Wikipedia
Does Copper Carbonate Dissolve Water Quora
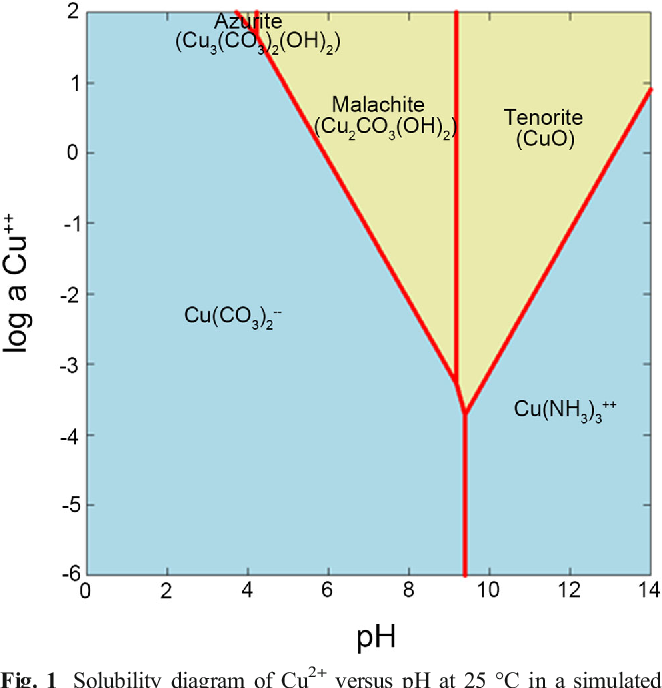
Copper Carbonate Semantic Scholar
