It is used to increase the. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element.

Molar Mass Molecular Weight Of Bacl2 Barium Chloride Youtube
Gas constant jaxlove260 jaxlove260 10052021 Chemistry High School answered expert verified Which measure of gas does the expression nRTP represent.

Molar mass of barium chloride. Gas constant 2 See answers Avolume Advertisement Advertisement okpalawalter8 okpalawalter8 We have that the expression nRTP. Youll determine the mass percentage of every element with these masses. Barium Fe3 ironIII Al3 aluminium Cr3 ChromiumIII F-fluoride Cl-chloride Br-bromide I-iodide HO-hydroxide NO 2-nitrite NO 3-nitrate HCO 3-hydrogencarbonate O2-oxide CO 3 2-carbonate SO 4 2-sulfate HSO 4-hydrogensulfate S2-sulphide N3-nitride PO 4 3 phosphate Use the ions given above to work out the formulae of the following ionic compounds.
This process is the main. Order EMSURE Quality Documentation. This calculator converts automatically the pressure to bar with the following conversion factor.
Iron III chloride hexahydrate is used as a coagulant for sewage and industrial wastes. The melting and boiling points of potassium chloride are 1040 K and 1690 K respectively. Solution for mass of H 2 S formed part b Now that we know the limiting reagent is water this problem becomes How much H 2 S is produced from 1000 g of H 2 O and excess aluminum sulfide 1 Determine moles of 1000 g of H 2 O water.
Percentage of mass solutes mass mass of solution x 100. BaOH 2 8H 2 O. This solution will be an unsaturated aqueous solution of sodium chloride.
Its toxicity limits its applicability. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
BaIO 3 2 H 2 O. Its density in the solid crystalline form is 1984 grams per cubic centimetre. In industry barium chloride is mainly used in the purification of brine solution in caustic chlorine plants and also in the manufacture of heat treatment salts case hardening of steel.
1 psi 6894810-2 bar. Remember the compound must have a net neutral. The preparation of BaNO32 by double conversion of barium chloride and sodium nitrate or calcium nitrate is described in the Eastern European patent literature.
25 g of NaCl s will dissolve in 100 mL of water at 25C. It is antagonistic to all muscle depressants no matter whether they act primarily on nerve or muscle. Number of moles C.
Sodium chloride MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents. Barium chloride along with. Our molar mass calculator has this for a variety of other compounds.
A student reacted 102 g of barium chloride with excess silver nitrate according to. After carefully removing any sulfur formed and the heavy metals the barium nitrate is crystallized at low temperatures. Sodium chloride NaCl is a soluble salt.
0 g of liquid bromine how many grams of aluminum would we need How many milliliters of 6. It is not incorrect to divide by the larger number and express the above ratios as 10213 and 02131 respectively. Initial stimulation of contraction leads to vasoconstriction through direct action on arterial muscle peristalsis through action on smooth muscle tremors and cramps through action on the skeletal muscles and.
K_sp is called solubility product constant or simply solubility productIn general the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. It is used as a component in oil well drilling fluids. Heres an example to better demonstrate the concept.
Chloride contents rarely attain 200 molL and the highest concentrations correspond to sam- ples located close to the sea Anse des Cascades and to two samples where anthropogenic pollution is suspected Les 3 Sources and Source Canal in the Rivire des Remparts ba- sin. The atomic mass is useful in chemistry when it is paired with the mole concept. 99710928 gmol Molar mass of perchloric is acid gmol Molar mass of CaCO3 is 100.
If you do not have a water analysis you can use the values given in the right column in the input table. From the solubility table above we see that the solubility of sodium chloride is 36 g100 mL water at 25C. It is used as a radiopaque agent.
The experimental molar ratio of I_2 to Al is 1470 Note. 3856 gcm 3 anhydrous 30979 g. 106404 View Pricing Availability.
Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Barium Sulfate Structure BaSO 4 Structure Barium Sulfate BaSO 4 Uses.
Molar Mass of Ammonium aluminium sulfate AlNH4SO42 Molar Mass of Ammonium nitrate NH4NO3 Molar Mass of Barium sulfate BaSO4 Molar Mass of Calcium carbonate CaCO3. The same concept can be extended to ionic compounds and molecules. It is just a matter of preference.
Finding Molar Mass for Other Chemical Compounds. These two contaminated samples also have high con- centrations of nitrates 25-105 molL whereas nitrate. The Mass percent formula is expressed as solving for the molar mass also for the mass of every element in 1 mole of the compound.
These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. The obtained osmotic pressure with formula 2 is in psi pounds per square inch. Barium stimulates striated cardiac and smooth muscle regardless of innervation.
1000 g 18015 gmol 0555093 mol 2 Use molar ratios to determine moles of H 2 S produced from above. Number of moles C. 20823 gmol anhydrous 24426 gmol dihydrate Appearance White solid Density.
1 g of NaCl s will dissolve in 100 mL of water at 25C. Mass percent components mass total mass x 100 or. The crystals of potassium chloride are made up of face-centred cubic FCC unit cells.
5844 gmol Chemical Formula. It is used in the casting of copper anode plates as a coating material. The molar mass of KCl is 745513 gramsmol.
GoldIII Chloride B2H6 Diborane B2O3 Boron Oxide B5H9 Pentaborane BaNO32 Barium Nitrate BaOH2 Barium Hydroxide BaCl2 Barium Chloride BaCO3 Barium Carbonate BaI2 Barium Iodide BaO Barium Oxide BaO2 Barium Peroxide BaSO4 Barium Sulfate BBr3 Boron Tribromide BCl3 Boron Trichloride BeCl2 Beryllium Chloride BeI2 Beryllium Iodide BF3 Boron. Based on the chemical formula of a substance we know the composition of the substance. Since both barium chloride and barium sulfate contain one mole of barium atoms pert mole of compound the moles of barium sulfate will be the same 0100 when barium has the limiting.
Sodium chloride carbon dioxide sulfuric acid glucose. A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present in the substance. Molar mass of al2s3.
Molecular Weight Molar Mass. How do you know the Order of Elements in a Chemical Formula What is Chemical Formula. Mass Amount Half-Life Emissions percent hr kghr Air 6.
One formula unit of sodium chloride. Lets consider the saturated solution of silver chloride AgCl.
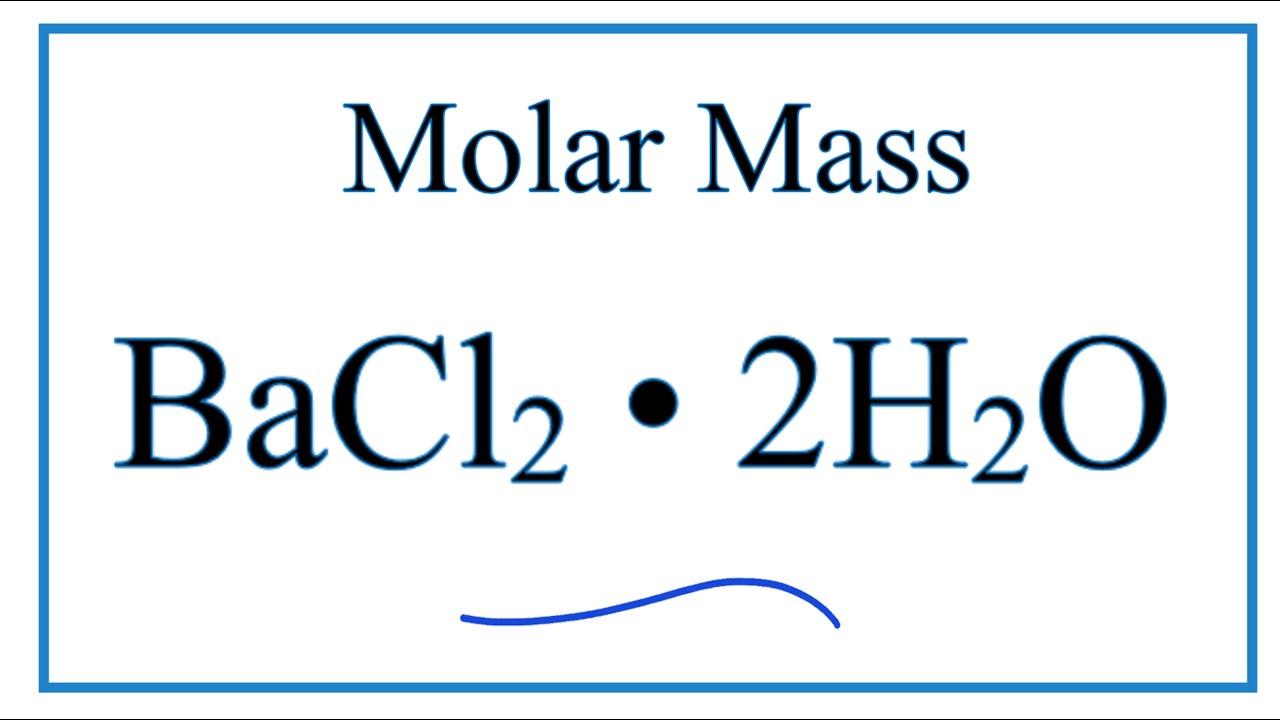
Molar Mass Molecular Weight Of Bacl2 2h2o Youtube

The Mole And Stoichiometry Ppt Download

Atoms In Barium Chloride Sample Youtube

Molar Mass Molecular Weight Of Bacl2 Barium Chloride Youtube

Molar Mass Molecular Weight Of Bacl2 2h2o Youtube

What Mass Of Barium Chloride Is Needed To Prepare 250 0 Ml O Clutch Prep

Chapter 3 The Atom Ppt Download

Stoichiometry Formulas And The Mole Lincoln High School Ppt Download
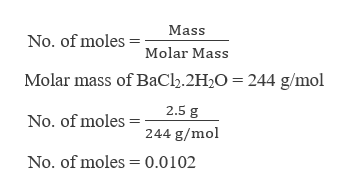
Answered What Is The Molarity Of A Barium Bartleby