Resins have a strong tendency to associate with asphaltenes and they help reduce the asphaltene aggregation. The chlorides bromides and iodides of all metals except lead silver and mercuryI are soluble in water.
Mercurous Sulfate Hg2so4 Pubchem
Asphaltenes and resins are heterocompounds and form the most polar fraction of crude oil.

Mercury sulfate solubility. S stands for Silver Ag. This means PbCl 2 is insoluble and form a precipitate. Another method of Au extraction uses cyanide in a two-stage process.
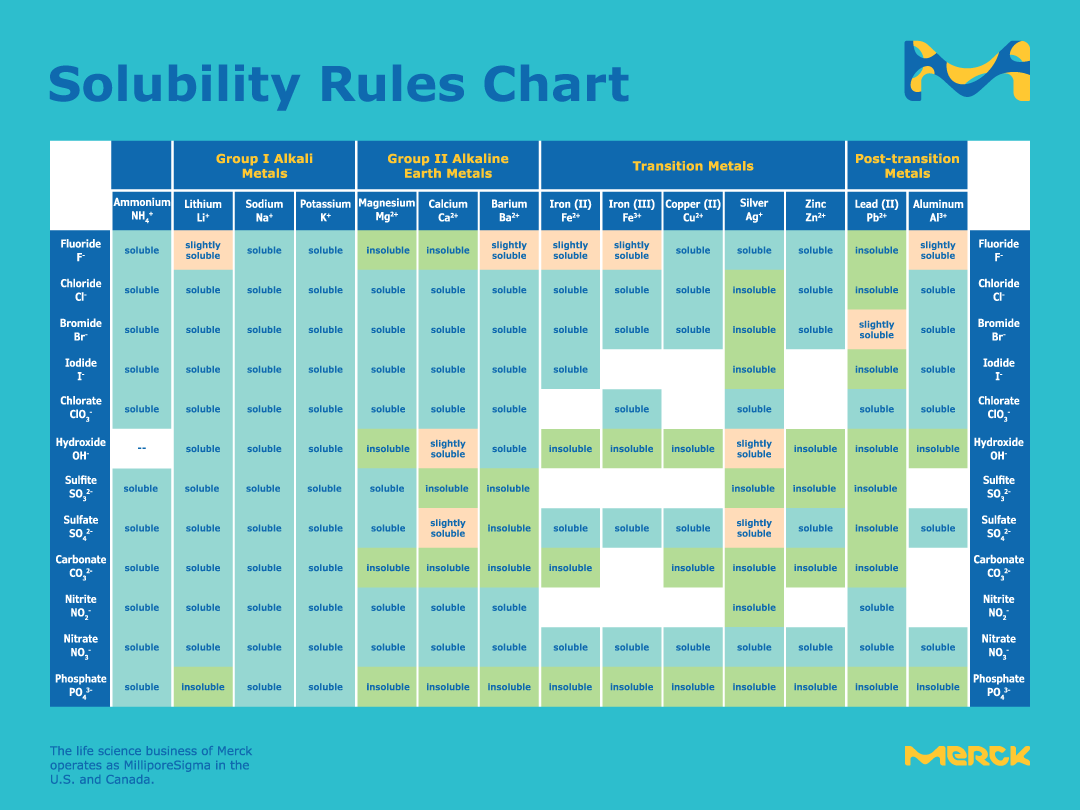
For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Solubility and Related Thermodynamic Quantities of CadmiumII Carbonate in Aqueous Systems JPCRD 2011 40 043104. Chlorides are soluble in water with the exception of silver lead and mercury.
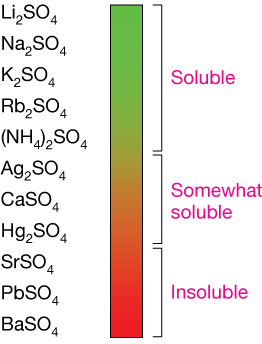
These 3 ions are never soluble with the sulfate group or group 17 nonmetals. Solubility Product Constants near 25 C. Note that silver sulfate and calcium sulfate dissolve just enough.
It is one of the simple methods to purify water. The solubility rules the technique ie when the product of ion concentrations in simple in the solution over the solubility product of the respective solid the precipitation occurs. The number of moles of a.
Ionic Compound Formula K sp. Henrys law states that the solubility of a gas is directly proportional to the partial pressure of the gas. The Solubility Rules 1.
Method II Semi-Micro Non-Volatile Residue. The finished reaction is. When writing out the mnemonic put a star next to PMS and a similar star next to the S and G of SAG to remember these are exceptions.
Strontium Sr 2 barium Ba 2 lead Pb 2 silver Ag calcium Ca 2 radium Ra 2 and diatomic silver Ag 2 2. Asphaltene solubility parameter can also be affected by other components in the oil like resins Hirschberg et al 1984. All sodium potassium and ammonium salts are soluble in water.
Please read our Terms Conditions and Privacy Policy for information about. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. The plasma and urine were.
Hg 2 SCN 2. Boiling point - the temperature at which a liquid turns into a gas. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3.
Silver acetate is sparingly soluble. But even though concentrations of MeHg in the. On the other hand if normal alkane pentane or heptane liquids are.
For more Solubility Complete data for COPPERII SULFATE PENTAHYDRATE 8 total please visit the HSDB record page. The treated water is reused whereas the settled portion is. Determination of Mercury by Cold Vapour Atomic Absorption Technique.
The nitrates chlorates and acetates of all metals are soluble in water. Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution. We would like to show you a description here but the site wont allow us.
Jitka Eysseltováa and Roger Bouaziz Potassium Sulfate in Water JPCRD 2012 41 013103. HgI2 is insoluble in water. Pb 2 and mercury II Hg 2 salts.
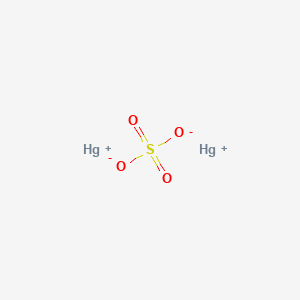
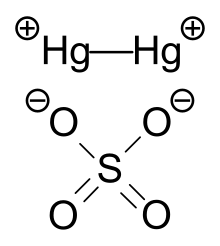
Hg 2 SO 4. Temperature affects the solubility of both solids and gases but hasnt been found to have a defined impact on the solubility of liquids. Ksp solubility product constants of many popular salts at SolubilityOFthings.
All are soluble except lead II Pb 2 barium Ba 2 and calcium Ca 2. Castro Bear may sound silly but it stands for the 3 ions. All are soluble except magnesium Mg 2 calcium Ca 2 strontium Sr.
The separations were carried out on a Synergi 10μ Hydro RP 80250 46 mm column using a 20 mM potassium phosphate buffer pH 65 for the plasma and 147 mM potassium phosphate23 mM tetrabutyl ammonium sulfate pH 50 for the urine with detection by absorption at 210 nm. MercuryII sulfide red HgS. The table below provides information on the variation of solubility of different substances mostly inorganic compounds in water with temperature at one atmosphere pressureUnits of solubility are given in grams per 100 millilitres of water g100 mL unless shown otherwise.
Those data indicate that the in environmental tracer studies measurement of monomethyl- stream emanating from the wetland was the major source of mercury CH3Hg by isotope dilution ICP-MS and detection of water DOC and sulfate to the lake during the year of this species transformation. MercuryII sulfide black HgS. Nitrogen Determination Kjeldahl Method Method I.
Remember Castro Bear to represent the second exception. 2 KClaq PbNO 3 2 aq 2 KNO 3 aq PbCl 2 s The solubility rules are a useful guideline to predict whether a compound will dissolve or form a precipitate. M stands for Mercury Hg 2 2.
The substances are listed in alphabetical order. MercuryII chloride or mercuric chloride historically also known as corrosive sublimate is the chemical compound of mercury and chlorine with the formula HgCl 2. Once used as a treatment for syphilis it is no longer used for medicinal purposes because of mercury toxicity and the.
All are insoluble except those of potassium K sodium Na and ammonium NH 4. The sulfate ion generally forms soluble compounds but there are several exceptions. Pressure can also affect solubility but only for gases that are in liquids.
Sulfates SO 4 2-. Phosphorous calcium magnesium and aluminium determination by Inductively Coupled Plasma-Atomic Emission Spectrophotometry ICP. Melting point - the temperature at which a solid turns into a liquid.
Globally ASGM is the second largest source of atmospheric mercury pollution after coal combustion. For both samples protein was precipitated out before injection using acetonitrile. Mercury amalgamation was the initial method used for centuries to process gold and is still in use today by artisanal and small-scale gold mining ASGM.
Hazardous Substances Data Bank HSDB Solubility in water g100ml at 0 C. It is white crystalline solid and is a laboratory reagent and a molecular compound that is very toxic to humans. Phosphate Determination as P 2 O 5.
This website uses cookies to help provide you with the best possible online experience. Carbonates CO 3 2-. The sulfate ion forms insoluble compounds with the following ions.
The chemicals are added to form particles which settle and remove contaminants from water. PbCl2 PbBr2 and PbI2 are soluble in hot water.
Mercury I Sulfate Hg2o4s Chemspider
Solubility Rules Solubility Of Common Ionic Compounds

Is Hgso4 Mercury Ii Sulfate Soluble Or Insoluble In Water
Mercury Ii Sulfate Hgo4s Chemspider

Mercury I Sulfate 97 Thermo Scientific Other Inorganic Compounds Chemicals Fisher Scientific