Specify a facility by using any combination of facility name and geographic location. P-methylaminophenol icsc triethoxysilane icsc 2-mercaptoimidazoline icsc ethylene glycol dibutyl ether icsc n-butyl ether icsc diethylene glycol diethyl ether icsc methylal icsc isopentane icsc melamine icsc 135-trimethylbenzene icsc acetophenone icsc phorone icsc tepp.
The designation of amines as primary secondary and tertiary is different from the usage of these terms in.

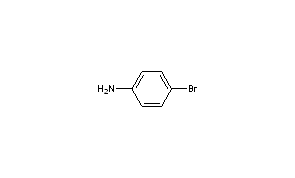
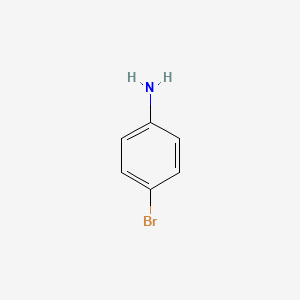
P- bromo aniline. You can use any reagent. The SEMS Search allows you to retrieve Superfund data from the Superfund Enterprise Management System SEMS database in Envirofacts. Benzenes o m p typically used for positions of substituents.
83762 free acid basis Compare Product No. The p-bromo acetanilide is obtained as colourless crystals mp. The main target users are workers and those responsible for occupational safety and health.
We offer 1-Bromo-3-Chloropropane 1-Pentyl Bromide 11-Bromoundecanoic Acid 4-Bromo Chlorobenzene 4-Phenyl Butyl Bromide and many more chemical compounds. Formally a carbanion is the conjugate base of a carbon acid. Several assessments are included with the guidelines models databases state-based RSL Tables local contacts and framework documents used to perform these assessments.
2-phenyloctane phenyl is attached at the second position of the longest carbon chain octane. C 25 H 42 N 7 O 17 P 3 S xLi CAS No. Bromination of both phenol and aniline is difficult to control with di- and tri-bromo products forming readily.
Amines are organic derivatives of ammonia in which one two or all three of the hydrogens of ammonia are replaced by organic groups. Because of their high nucleophilic reactivity aniline and phenol undergo substitution reactions with iodine a halogen that is normally unreactive with benzene derivatives. How can we form o-bromo-Aniline or p-bromo-aniline is using anilne.
Molecular formula of acetanilide C 8 H 9 NO. Toluene Phenol Benzaldehyde Benzoic acid Aniline m-chloronitrobenzene p-nitrophenol H2 H3CCBr CH3 OH CH O C O OH NH2 NO2 Cl OH O2N. Combined wastewater streams generated from nitrobenzeneaniline production T K105.
We are a manufacturer suppliers of bromine based chemicals Aliphatic Bromide based at Vadodara Gujarat India. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Wiley-Interscience Wiley Sons Inc.
Bromination of both phenol and aniline is difficult to control with di- and tri-bromo products forming readily. Putting it all together the name can be derived as. The corresponding statement to each P-code can be found at the GHS Classification page.
UV-Vis and fluorescence spectroscopies show that in these non-covalent hybrids the polymer forms p-stacked. Icsc tetrahydrofurfuryl alcohol icsc. How the EPA conducts risk assessment to protect human health and the environment.
Weight of p-bromo acetanilide 214 gmole. This copolymer can functionalize single-walled carbon nanotubes SWNTs non-covalently and disperse more SWNTs than its hexyl analogues. Dielectric Constant Tablexls Aniline 68 F 73 Aniline 212 F 55 Aniline Formaldehyde Resin 35 - 36 Aniline Resin 34-38 Anisaldehyde 68 F 158 Anisaldoxine 145 F 92 Anisole 68 F 43 Anitmony Trichloride 53 Antimony Pentachloride 68 F 32 Antimony Tribromide 212 F 209 Antimony Trichloride 166 F 330 Antimony.
We describe the synthesis of a copolymer of dodecylthiophene and its analogue bearing an aniline group at the end of the dodecyl side chain. Separated aqueous stream from the reactor product washing step in the production of chlorobenzenes T K107. Reacts explosively with aniline diallyl sulfide and hydrazine hydrate.
The mixed halogen iodine chloride ICl provides a more electrophilic iodine moiety and is effective in. The primary aim of the cards is to promote the safe use of chemicals in the workplace. Bn similar to the phenyl group is formed by manipulating the benzene ring.
Empirical Formula Hill Notation. In the case of the benzyl group it is formed. Ed Saxs Dangerous Properties of Industrial Materials.
List Of Known Azeotropic Mixtures. The Sandmeyer reaction provides a method through which one can perform unique transformations on benzene such as halogenation cyanation trifluoromethylation and hydroxylation. I was thinking to react Aniline with Br2 in presence of Ch3ch2-Oh But I dont know the mechanism also Im confuse will this really give the desired product In my textbook they reacted aniline first with an aldehyde and then with bromine water.
The mixed halogen iodine chloride ICl provides a more electrophilic iodine moiety and is effective in. Weight of acetanilide 135 gmole. This section is from the book Distillation Principles And Processes by Sydney YoungAlso available from Amazon.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Where B stands for the base.
Molecular formula of pbromo acetanilide C 8 H 8 NOBr. Process residues from aniline extraction from the production of aniline T K104. Because of their high nucleophilic reactivity aniline and phenol undergo substitution reactions with iodine a halogen that is normally unreactive with benzene derivatives.
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. The carbanions formed from deprotonation of alkanes at an sp 3 carbon alkenes at an sp 2 carbon arenes. Hazardous Substances Data Bank HSDB Incompatible materials.
In this chapter unless the context otherwise requires. 14- across from each other in a benzene ring. The benzyl group abbv.
Aniline 32 F 78 Page 3 6242011. Distillation Principles And Processes. Administer means to apply inject or facilitate the inhalation or ingestion of a substance to the body of a person.
It is an example of a radical-nucleophilic aromatic substitution. Unless specifically excepted or unless listed in another schedule or contained within a pharmaceutical product approved by the United States Food and Drug Administration any material compound mixture or preparation that contains any quantity of a synthetic cannabinoid found to be in any of the following chemical class descriptions or homologues nitrogen. Compounds RNH 2 are called primary amines R 2 NH secondary amines and R 3 N are tertiary amines.
Predict the product of the following reaction. Also Give structures of intermediate. The ICSC project is a common undertaking between the World Health Organization WHO and.
Column bottoms from product separation from the production of 11-dimethylhydrazine UDMH from. A carbanion is an anion in which carbon is trivalent forms three bonds and bears a formal negative charge in at least one significant resonance form. R 3 CH B R 3 C.
Nitrobutyl morpholine ethylnitro-trimethylene dimorpholi 12-benzisothiazoline-3-one sodium salt 13-butandiol-dimethacrylate 13-diphenylguanidine 135-tris-2-hydroxyethyl-hexahydrotriazine grotan bk 14-butandioldimethacrylat budma 14-butanedioldiglycidyl ether 16-hexanediolediglycidyl ether 2-2-aminoethoxy-eth 2-bromo-2nitropropane-1 3-diol bronopol 2-ethylhexyl. O- m- p-Common names for substituted benzenes are often used accepted by IUPAC. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem.
135 g of acetanilide gives 214 g of p-bromo.

Preparation Of P Bromo Aniline Youtube
4 Bromoaniline C6h6brn Chemspider
4 Bromoaniline Brc6h4nh2 Pubchem
4 Bromo N N Dimethylaniline C8h10brn Pubchem

4 Bromoaniline 98 Thermo Scientific Halobenzenes Benzene And Substituted Derivatives Fisher Scientific

4 Bromoaniline 106 40 1 Tokyo Chemical Industry Co Ltd Apac