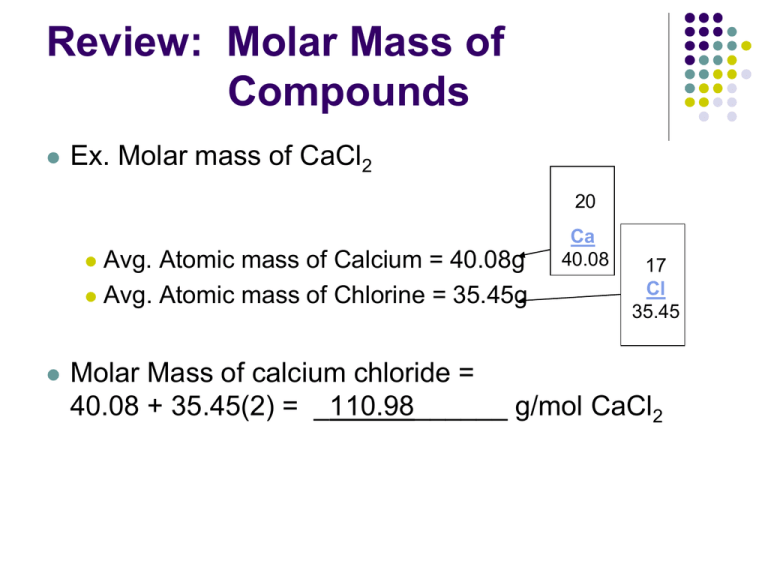
This way we can calculate the molar mass of a compound or one-carbon compound. If the molar mass of the salt is 218 gmol what mass is required.

A Sample Of Calcium Bromide Contains 0 2 G Calcium And 0 8 G Bromine By Mass Calculate The Empirical Brainly Com
Molar Mass of Frequently Calculated Chemicals.
Molar mass of calcium bromide. Well add those numbers together along with the unit grams per mole for finding molar mass. Based on the chemical formula of a substance we know the composition of the substance. Since sodium carbonate contains one carbon atom two sodium atoms and three oxygen atoms the molecular weight is.
Atomic mass of Carbon 1201. What is the formula mass amu of calcium phosphate. The dissolution stoichiometry shows a 11 relation between moles of calcium ion in solution and moles of compound dissolved and so the molar solubility of CaOH 2 is 69 10 3 M.
The identity of a substance is defined not only by the types of atoms or ions it contains but by the quantity of each type of atom or ion. Check Your Learning The K sp of PbI 2 is 14 10 8. How do you know the Order of Elements in a Chemical Formula What is Chemical Formula.
Use the value ksp14x10-8 for PbI2 to solve the following problems. What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml. Molar Mass Is 9896G.
Atomic mass of Oxygen 1600. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula.
230 x 2 46. Carbon2427 12 202. Molecular mass or molar mass are used in stoichiometry calculations in chemistry.
Molar absorptivity of all-trans retinol in ethanol at 325 nm is ɛ 52770 M 1 cm 1. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Molar absorptivity of all-trans retinoic acid at 350 nm in ethanol ɛ 45300 M 1 cm 1.
For example water H 2 O and hydrogen peroxide H 2 O 2 are. These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. What is the molar mass of sodium carbonate Na2CO3.
Now we have to divide all the values with the lowest obtained value. It is defined to be 112 of the mass of one atom of carbon-12 and in older works is also abbreviated as amu. Individual compounds include the anhydrous material x 0 the hexahydrate x 6 and the rare dihydrate x 2.
How many grams are in 379 moles of calcium bromide CaBr 2. Molar mass of Carbon Monoxide 2801. Calculate the molar mass of.
How many grams of H_3PO_4. If you need a 15 M solution of calcium bromide eqCaBr_2 eq and have 850 grams of solid eqCaBr_2 eq how many milliliters of solution can you make. Calcium bromide is the name for compounds with the chemical formula Ca Br 2 H 2 O x.
To Calculate Empirical Formulae first we have to divide the given percentages of atoms by their molecular masses. Path length L 1 cm for standard cuvette. That leads to this.
Click here to see a video of the. Hydrogen 407 1 407. All are white powders that dissolve in water and from these solutions crystallizes the hexahydrate.
The standard molar entropy associated with calcium oxide corresponds to 40 joules per mole kelvin. As defined in the ICE table x is the molarity of calcium ion in the saturated solution. In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses.
What is the mass solubility of calcium sulfate in pure water expressed in gL. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Ksp24x10-5 for calcium sulfate.
This compound is known to emit an intense glow when it is heated to temperatures above 2400 degrees celsius. How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution. 120 x 1 12.
Hydrogen 407 201 2. If molecular formula calculator add up the total value which is 12 46 48 106. CaOH 2 SO 4 CaSO 4 H 2 O.
Will precipitation occur when you add 005 mL of 010 M KBr to a saturated solution of AgCl. Also important in this field is Avogadros number N. MM millimolar millimoles per litre 10-3 moles per litre µM micromolar micromoles per litre 10-6 moles per litre nM nanomolar nannomoles per litre 10-9 moles per litre pM picomolar picomoles per litre 10-12 moles per litre fM femtomolar femtomoles per litre 10-15 moles per litre.
What is the molar solubility of calcium sulfate in pure water. A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present in the substance. Calcium phosphate Ca 3 PO 4 2 is an ionic compound and a common anti-caking agent added to food products.
Chlorine7165 355 201. λ max of all-trans-retinoic acid in ethanol is 350 nm. We can also use molecular weight calculator for finding molar mass of a.
Soluble in water glycerol. A solution of calcium bromide contains 200 g dm-3. Therefore the molar mass of Na2CO3 is 106 gmol.
16 x 3 48. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. A the anesthetic halothane C 2 HBrClF 3 b the herbicide paraquat C 12 H 14 N 2 Cl 2 c caffeine C 8 H 10 N 4 O 2 d urea CONH 2 2 e a typical soap C 17 H 35 CO 2 Na.
The hydrated form is mainly used in some drilling fluids. What is the molar. How many grams are in 0572 moles of glucose C 6 H 12 O 6.
Calculate the molar mass of each of the following. MV mass molar mass x 100 L 200 g 199886 gmol x 0100 M When CaBr 2 ionizes two bromide ions are released for every one CaBr2 that dissolves. In the NaOH.
Now use the number of moles and multiply it by the molar mass. Molarity moles per litre of solution M Commonly used terms include. Carbon202 2.
What volume of 12 M HCl solution is needed to prepare 5 liters of 00250 M solution. What is the molarity of the solution with respect to calcium bromide and bromine ions.

How Many Molecules Are In 5 0 Grams Of Cal Clutch Prep

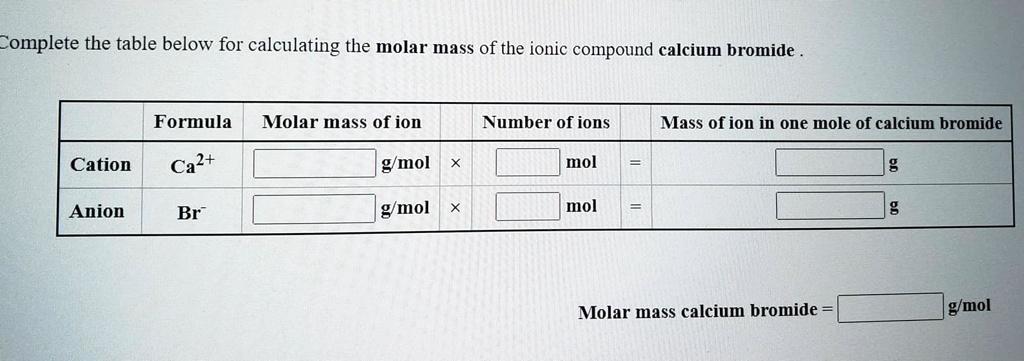
Solved Complete The Table Below For Calculating The Molar Mass Of The Ionic Compound Calcium Bromide Formula Molar Mass Of Ion Cation Ca2 G Mol Number Of Ions Mass Of Ion In One Mole
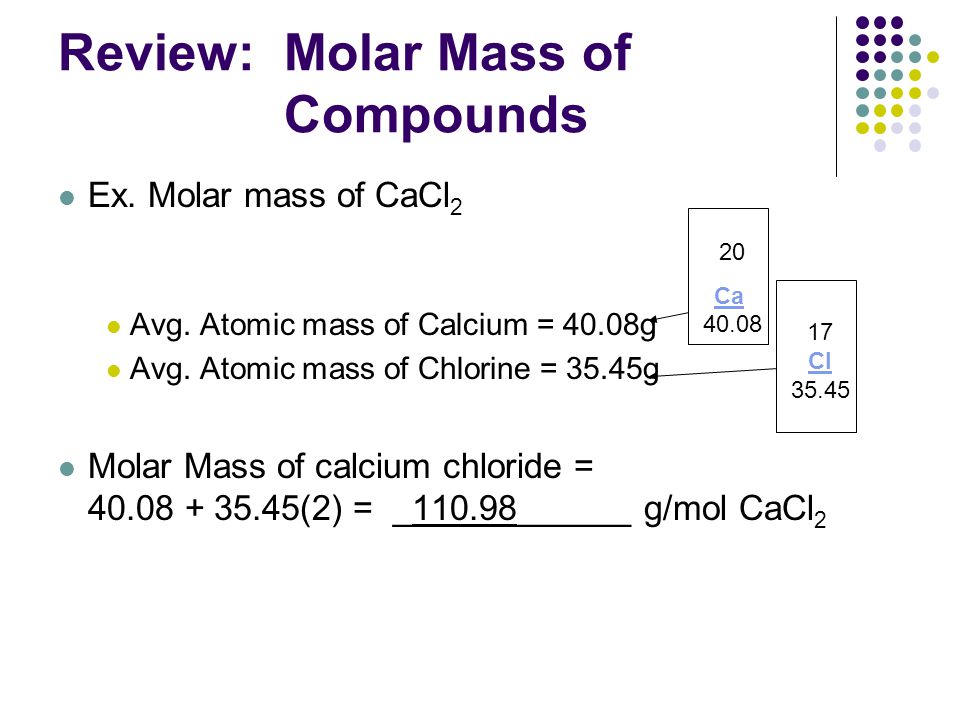
Review Molar Mass Of Compounds Ppt Video Online Download
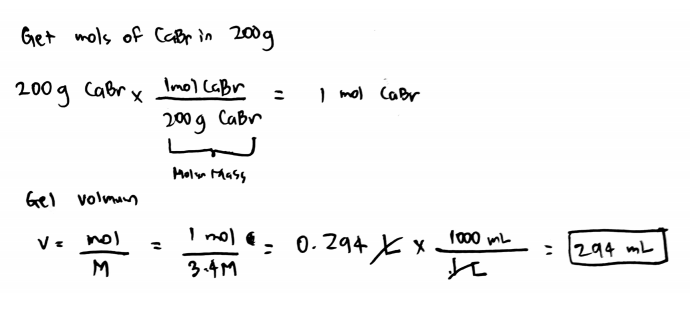
Calculate The Volume In Millimeters Of A 3 Clutch Prep

How To Find The Number Of Atoms In Cabr2 Calcium Bromide Youtube

Molar Mass Molecular Weight Of Cacl2 Youtube

Section 3 5 Counting Molecules Ppt Download