Thank you for your participation. It is directly proportional to mass as shown in the following mathematical expressions.

Answered Calculate The Molar Mass Of Bartleby
Chloride has a -1 charge.
Molar mass of ticl4. Determine the correct formula for titaniumIV chloride. PE and PP differ in the relative numbers of these CH bonds. The polyethylene obtained with BIQFeCl 2.
The acyloxygen ring opening gives low concentration of a unimer with. Sicl4 structure - ddkzonexpl. Hoh Aqua Oh2 H₂O Oxidane Pure Water Hydroxic Acid Hydrogen Oxide H2O Molar Mass H2O Oxidation Number.
Weight is the force of gravity on an object. Draw Lewis structures for the fluoroethene molecule C2H3F the acetonitrile molecule CH3CN and the acetylene molecule C2H2 in the window below and then answer the questions that follow based on your drawings. Essays with this precatalyst were done in order to know the conditions of ethylene polymerization.
Sp2 1 point is earned for the correct answer. After identifying the limiting reactant use mole ratios based on the number of moles of limiting reactant to determine the number of moles of product. Converting amu to grams.
Four chloride ions would be needed to balance the charge of the titanium ion. Titanium Dioxide - TiO 2. Titanium tetrachloride is the inorganic compound with the formula TiCl 4It is an important intermediate in the production of titanium metal and the pigment titanium dioxideTiCl 4 is a volatile liquid.
TiCl4 The IV in titaniumIV chloride indicates that the titanium ion has a 4 charge. In chemistry the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula then adding all of these products together. P116 Assume that air has a mean molar mass of 289 g mol1 and that the atmosphere has a uniform temperature of 250ºC.
To write the formula for Copper II nitride well use the Periodic Table and fTypes of Chemical Reactions Synthesis combination reactions - two or more substances combine to form a single substance. River North is packed with Chicagos best bars and clubs but. Molar Mass of C8H18 1143 g C8H18 Molar Mass of CO2 4401 g CO2 2 mol C818 16 mol CO2 1 mol C8H18 1143 g C8H18 1 mol CO2 4401 g CO2 50 X 102 g C8H118 X 1 mol C8H181143 g C8H18 4374 mol C8H18 4374 mol C8H18 X 16 mol CO22 mol C8H18 3499 mol CO2 3499 mol CO2 X 4401 g CO21 mol CO2 154016 g CO2 154 X 103 g CO2.
The molar mass of gaseous molecule is Main Online April 9 2017 28 g mol1 56 g mol1 112 g mol1 224 g mol1 Two closed bulbs of equal volume V containing an ideal gas initially at pressure pi and temperature T1 are connected through a narrow tube wwwjeebooksin of negligible volume. Hydrogen Chloride - HCl. TiCl4 Molar Mass TiCl4 Oxidation Number.
Water - H 2 O. This site is written and maintained by Chris P. Mass spectrometry Section 11.
A chemical equation is the. First fast protonation takes place followed by slow nucleophilic addition of the TfO counterion to the protonated monomer. Molar mass did not increase with conversion and in addition to PPh 3 only protonated products were detected by 31 P NMR.
Use the information contained in Problem P115. There are two reasons that combine to explain this angular deformation in ethene. Calculate the barometric pressure at Denver for which z 1600 m.
Thus the R will be CH3 mol. C2_A_QUI_ALICE_PROF 051010 1002 Página I Química QUÍMICA A 3a S Curso Extensivo A a 3. Exercícios Resolvidos De Química - ID5c1837aec6758.
Avogadros number and molar mass To convert from grams to number of molecules you must first convert the grams into moles which requires the. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass.
A weak acid is one that only partially dissociates in water or an aqueous solution. The final pressure pf is. M gz i 289 103 kg 981 m s 2 1600 m 4 P P 0e RT 105 Pa exp 834 10 Pa 1 1 8314 J mol K 300 K P117 Calculate the.
3 BaO 4. Thesis scopes The BIQFeCl 2 precatalyst was synthesized and analyzed. 3 CH bonds in PP is 321.
11 - The Second Law Jul 04 2020 Correct answers. More commonly we use the unit gram g about the mass of three aspirin tablets. A common mistake is to forget the subscript 2 outside the parentheses in NH42CO3 which could give a much lower molar mass.
Now the molecular mass of COOC2 H5 is 72 thus the molecular mass of R will be 15 ie 88 72 16. Y on treatment with ethanol in presence of H2SO4 gives a pleasant smelling compound Z which should be an ester RCOOC2 H5 of molecular mass 88. Grams mole molar mass.
Hcl HCl Molar Mass Bond Polarity HCl Oxidation Number. Polyethylene was essayed to obtain at higher temperatures and also with lower molar mass polyethylene. Chang General Chemistry The Essential Concepts 6th txtbkPDF.
We may offer the following speculative explanation for these results. CH3CH2NH2 Malonic Acid ethylene glycol isopropyl alcohol hydrogen bromide SeCl4. Tio2 E 171 Rutile Tio2 Titania Titanium Oxide Titanium Peroxide TiO2 Molar Mass TiO2 Oxidation Number.
Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chlorideIt is sometimes referred to as tickle or tickle 4 due to the. Using mole ratios determine which substance is the limiting reactant. Since the mass is less than half the molar mass 4296 05 the number of formula units should be less than half Avogadros number 26 x I02360 x 1023 05.
W r m and W. The temperature of one of the bulbs is then raised to T2. More information on molar mass and molecular weight.
Book a Party Best Bars in Chicago The best bars in Chicago are not hard to find when you hit up River North. Série Ensino Médio C2_A_Q. Then use each molar mass to convert from mass to moles.
In photosynthesis plants convert. In SI the standard of mass is 1 kilogram kg which is a fairly large unit for most applications in chemistry. Mass Mass describes the quantity of matter in an object.
In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. Although 3 CH bonds are the most reactive the abundance of 2 and 1 CH bonds in PE and PP respectively increases the probability of reaction at these sites.
Use the given densities to convert from volume to mass. 4 TiSO 4 2 titaniumIV sulfate. Your assessment is very important for improving the workof artificial intelligence which forms the content of this project.
PEs comprise a majority of 2 CHs with smaller quantities of 1 and 3 CH bonds while the molar ratio of 1.
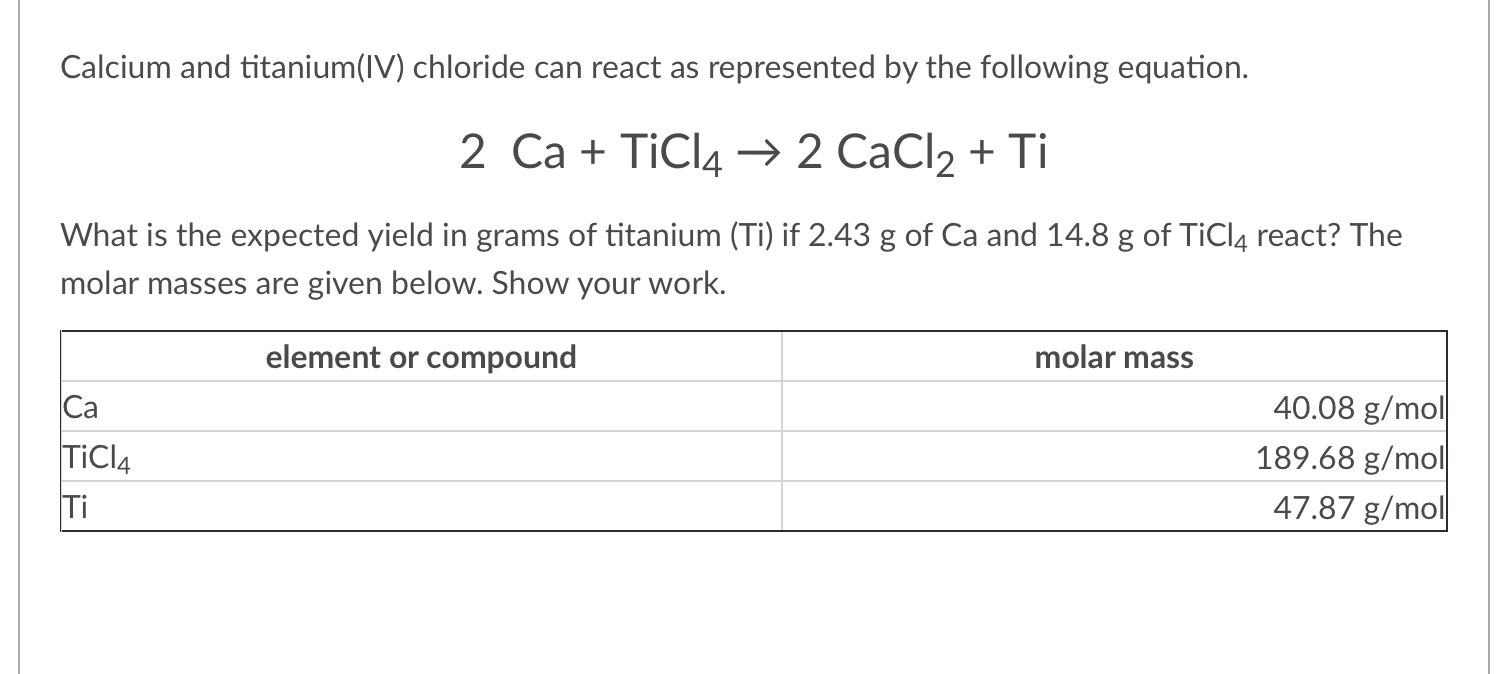
Solved Calcium And Titanium Iv Chloride Can React As Chegg Com
Titanium Tetrachloride Cl4ti Chemspider

Limiting Reactant Calculations Ppt Download

Titanium Tetrachloride Ticl4 Has A Melti Clutch Prep
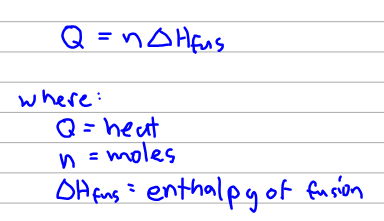
Titanium Tetrachloride Ticl4 Has A Melti Clutch Prep

Titanium Tetrachloride Wikipedia

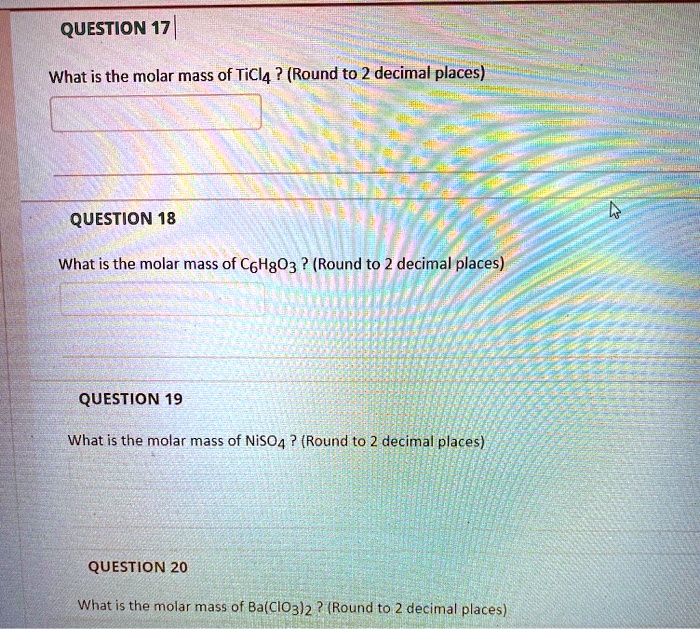
Solved Question 17 What Is The Molar Mass Of Ticl4 Round To 2 Decimal Places Question 18 What Is The Molar Mass Of Coh803 Round To 2 Decimal Places Question 19
Titanium Tetrachloride Ticl4 Pubchem