Autooxidation results in hydroperoxide formation side-chain cleavage and eventually formation of short chain acids such as formic acid all of which could influence the stability of a biopharmaceutical product. Both PEG and PEO have chemical similarities and can be photo-cross-linked by modifying the polymer chain end with acrylates or methacrylates.
This reactivity also includes organic structures within cells and cell nuclei.

Ethylene oxide structure. In this case alkylation and reactions with DNA RNA and proteins occur. The respective half lives were 025 and 2 hours. Ethylene glycol has been synthesized by the oxidation of ethylene with O 2 to ethylene oxide and the subsequent hydration of ethylene oxide to ethylene glycol.
Usually ethylene is supplied from the thermal cracking of naptha from petroleum refining. Nickel single atom and copper nanoparticles used for highly selective tandem electrocatalysis of CO2 to ethylene. Ethylene oxide is used as an intermediate in the production of ethylene glycol and nonionic surfactants.
The tandem electroreduction of CO2 to. Ethylene is the starting material for the preparation of a number of two-carbon compounds including ethanol industrial alcohol ethylene oxide converted to ethylene glycol for antifreeze and polyester fibres and films acetaldehyde converted to acetic acid and. The new research study on Ethylene Oxide market sheds light on the current scope as well as on the upcoming opportunities in the future.
Ethylene oxide is a substance which due to its structure is counted among the very reactive compounds. All these six atoms H-C-H form an angle of 1174 close to the 120 to form a hybridized carbon sp². In English 5035 7988-7997 2011 Non-fouling surfaces produced by gas phase pulsed plasma polymerization of an ultra low molecular weight ethylene oxide containing monomer.
Hydrogel formation can be initiated by either crosslinking PEG by ionizing radiation or by covalent crosslinking of PEG macromers with reactive chain ends. Ethylene glycol is widely used as antifreeze in automobile cooling systems and in the manufacture of human-made fibres low-freezing explosives and brake fluid. The report also focuses on global major leading industry players of Ethylene Oxide market providing information such as.
By Liu Jia Chinese Academy of Sciences. The peak blood ethylene-glycol concentration in ethylene-glycol dosed rats was 11. The peaks at 471 ppm peak d and 364 ppm peak h are assigned to the protons from ethylene glycol units in PEF and methylene oxide units in PTMO segments while those at 721 ppm peak c to the aromatic protons from furan rings respectively.
Propylene glycol also called 12-propanediol resembles ethylene glycol in its physical. Angewandte Chemie International ed. Ethene is a hydrocarbon which has the formula C 2 H 4 or H 2 CCH 2It is a colorless flammable gas with a faint sweet and musky odor when pure.
When crosslinked into networks PEG can have high water content forming hydrogels. ISO 10993-72008 specifies allowable limits for residual ethylene oxide EO and ethylene chlorohydrin ECH in individual EO-sterilized medical devices procedures for the measurement of EO and ECH and methods for determining compliance so that devices may be released. Ethylene glycol is a prominent member of the.
Ethylene oxide found in PEG-4 PEG-7 PEG4-dilaurate and PEG 100 is highly toxiceven in small dosesand was used in World War I nerve gas. Because it is a strained ring ethylene oxide easily participates in a number of addition reactions that result in ring-opening. It is produced commercially from ethylene oxide which is obtained from ethylene.
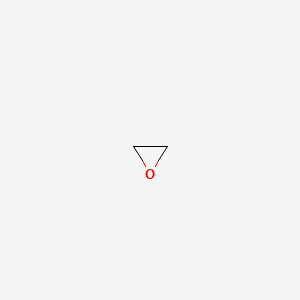
The molecular structure is based on structures generated from information available in ECHAs databases. Applicability of allowable limits for neonates and infants. Ethylene oxide is an organic compound with the formula C 2 H 4 OIt is a cyclic ether and the simplest epoxide.
Ethylene is widely used in the chemical industry and its worldwide production over 150 million tonnes in 2016 exceeds that of any other organic. And then there is 14-dioxane found in PEG-6 PEG-8 PEG-32 PEG-75 PEG-150 PEG-14M and PEG-20M which on top of being a known carcinogen may also combine with atmospheric oxygen to form explosive peroxidesnot exactly something you want. Cytotoxicity carcinogenicity and mutagenicity of ethylene oxide which have been demonstrated by many in vitro and in vivo tests are.
If generated an InChI string will also be generated and made available for searching. Further the bond is rigid about the C-C bond with high energy process by breaking the π-bond. Ethylene oxide sterilization residuals Amendment 1.
Preparation of Ethylene Glycol C 2 H 6 O 2. A three-membered ring consisting of one oxygen atom and two carbon atomsEthylene oxide is a colorless and flammable gas with a faintly sweet odor. Breathing in relatively high levels of ethylene oxide can cause irritation of the eyes skin and respiratory passages and affect the nervous system for example headaches nausea vomiting memory loss numbness in humans.
A small amount less than 1 is used to control insects in some stored agricultural products and a very small amount is used in hospitals to sterilize medical. Oxidation of the fatty acid moiety while well described in. This information is only displayed if the substance is well-defined its identity is not claimed confidential and there is sufficient information available in ECHAs databases for.
It is the simplest alkene a hydrocarbon with carbon-carbon double bonds. A small amount is used as a fumigant for. As the name suggests it has four atoms of hydrogen bonds that are paired with carbon atoms with a double bond.
The intra-cluster electron transfer towards Pd across the as-formed nanometer metaloxide interface significantly weakens the ethylene adsorption without compromising the. Wu YJ et al. Peak blood ethylene-carbonate and ethylene-glycol concentrations in rats dosed with ethylene-carbonate were 0028 and 23 umolg respectively.
Obermeier B et al. ISO 10993-72008Amd 12019 Biological evaluation of medical devices Part 7. It can be easily synthesized by the anionic ring opening polymerization of ethylene oxide into a range molecular weights and variety of end groups.
Ethylene is a hydrocarbon. For more than 30 years Polymer Engineering Science has been one of the most highly regarded journals in the field serving as a forum for authors of treatises on the cutting edge of polymer science and technology. 87121 Poly ethylene glycol and poly ethylene oxide PEG and PEO are currently FDA licensed and are used for several tissue engineering applications.
Additional background including guidance and a flowchart showing how the standard is applied are also included in. Ethylene oxide is a flammable gas with a somewhat sweet odor. Ethylene glycol and some of its derivatives are mildly toxic.
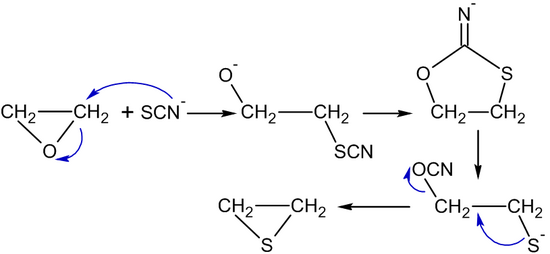
To understand the structure of global trading the report also gives statistical data on local consumption and global consumption. The polysorbates undergo autooxidation cleavage at the ethylene oxide subunits and hydrolysis of the fatty acid ester bond. It is very reactive with nucleophilic substances such as water alcohols halides amines and sulfhydryl compounds.
Ethylene oxide is a highly flammable gas produced in very large quantities in the United States 53 to 63 billion pounds. Ethylene-glycol was the only ethylene-carbonate metabolite detected. Long-term exposure to ethylene oxide at high levels encountered at some workplaces has also been associated with a small to moderate increase in the incidence of some.
It dissolves easily in waterEthylene oxide is a man-made chemical that is used primarily to make ethylene glycol a chemical used to make antifreeze and polyester. Multifunctional Poly ethylene glycols.

File Ethylene Oxide Chemical Structure Png Wikimedia Commons

File Ethylene Oxide Png Wikimedia Commons
Ethylene Oxide Gulf Coast Environmental Systems
Ethylene Oxide C2h4o Chemspider

Ethylene Oxide American Chemical Society
.jpg)