A ammonium nitrate B copperII chloride C ironII nitrate D ironIII chloride Jun 20 2015 NH 4 NO 3 NaOH NaNO 3 NH 3 H 2 O. Calculate the formula mass.
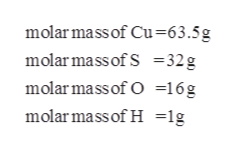
Answered Calculate The Molar Mass Of Cuso4 Bartleby
If you have crystalline reagent grade cupric sulfate you will get first-rate crystals.
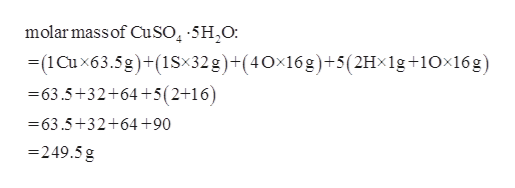
Copper sulfate hydrate molar mass. Single Replacement and Percent Yield Moles Lab Activity 7. LEAVE TUBE TIPPED DOWNWARD. Implementing POGIL in the Lecture and the Science.
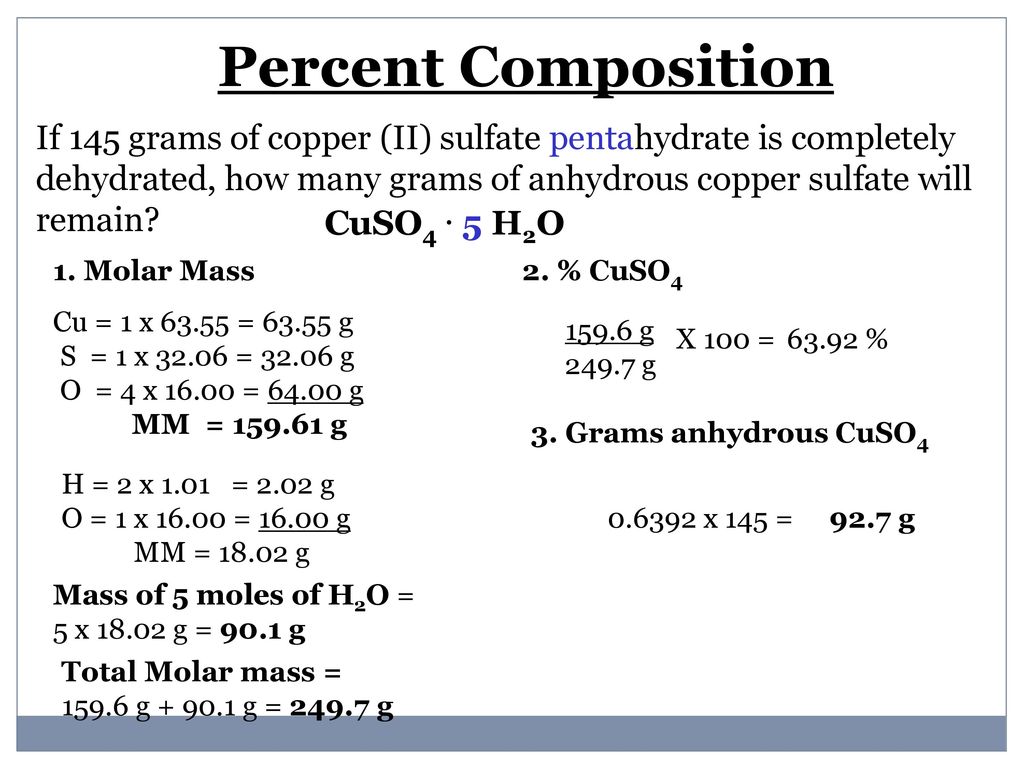
Impact of Learners Prior Knowledge on Their Use of Chemistry Computer Simulations. A hydrate is an ionic compound that has a definite amount of water molecules attached to its crystalline structure. 15960 gmol anhydrous 249685 gmol pentahydrate.
15 g Cu 1592 g Cu 2 S 1271 g Cu 188 g Cu 2 S Check. Students can get great results using copper sulfate. In the case of copperII sulfate hydrates you only get x as a whole.
Various forms of. TIP THE TEST TUBE SLIGHTLY DOWNWARD 2. Copper is surrounded by six oxygen atoms provided by two different sulfate groups and four molecules of water.
Convert grams of each into moles of each by dividing by molar mass 3. Determine the mass of water in the hydrate by subtracting the mass of the anhydrous from the mass of the hydrate if necessary -OR-convert s into grams by assuming a 100g sample 2. Remember that there are colorred5 moles of water for every 1 mole of hydrate 15961.
Mass of the empty crucible 400 g Mass of the crucible plus hydrate sample 450 g Mass of the system after heating 432 g Mass of the system after a second heating 432 g 36 a. Heating will drive off the water as a vapor in hydrated copperII sulfate. Many compounds exist as hydrates.
For a general form copperII sulfate hydrate formula CuSO_4 xH_2O we use x to represent the number of water molecules present as water of crystallization per formula unit of anhydrous copper II sulfate CuSO_4. Sulfuric acid copperII salt 11 pentahydrate. If the anhydrate of the hydrate were.
Synthesis of an Oxide of Copper Moles Lab Activity 6. Our unknown hydrate may be a hydrate of copperII sulfate magnesium sulfate ironIII chloride or ironIII nitrate. Divide the mass of water in one mole.
Our aim is to discover new pathways within and among these three modules and to identify points of vulnerability in human diseases. Molar mass of CuSO 4 15961gmol. The mass of copper sulfide formed will be determined by the mass of copper available.
Divide the mass of the water lost by the mass of hydrate and multiply by 100. 1 Copper II sulfate - Determining the Number of moles. The pentahydrate x 5 is the most common form.
Schroeder JD and Greenbowe TJ. Is this answer reasonable. Now you have to determine how many grams of copperII sulfate pentahydrate would contain this many grams of copperII sulfate.
1 Cu6355 gmol 1 S3207 gmol 4 O1600 gmol 15962 gmol Formula mass 15962 gmol 5 H 2 0 1802 g H 2 0mol 24972 gmol 2. Sulfate SO 4 2 Ca 2 Sr 2. 147g H 2 O x 1 mol H 2 O 1802g mol-1 H 2 O 008158 mol H 2 O - Ratio of Water to Anhydrate.
Put about ½ inch of hydrated copper sulfate crystals in a dry test tube. Have a team of editors who proofread every paper to make sure there are no grammar errors and typos. Some examples are copper II sulfate sodium sulfate nickel II sulfate iron II sulfate cobalt II chloride magnesium sulfate.
Record and calculate the following about the copper sulfate pentahydrate a mass of CuSO45H2O g b Molecular weight of CuSO45H2O cMoles of CuSO45H2O dMoles of Cu in the copper sul. Conservation of MassReaction of Vinegar and Baking Soda Moles Lab. Copper sulfate pentahydrate will grow beautiful blue rhombic crystals.
Formula Mass atomic mass unit amu The sum of the average atomic masses of all atoms represented in its formula i. A process during which two reactants form a precipitate Solutions of sodium hydroxide and hydrochloric acid are mixed. Copper 2 sulfate pentahydrate.
The ratio of water mol ecules to the anhydrous salt is constant. Molar mass of H 2 O 1802gmol. E mass of copper Average copper content f copper content 12B Exercise CopperII sulfate forms a hydrate which contains 361 by mass water.
Alka-Seltzer Moles Lab Activity 8. 148g CuSO 4 x 1 mol CuSO 4 15961g mol-1 CuSO 4 0009273 mol CuSO 4. Upper Saddle River NJ.
SolutionsAqueous Copper II Sulfate Pent hydrate SolutionsAlum Moles Lab Activity 5. The theoretical actual percent hydration percent water can be calculated from the formula of the hydrate by dividing the mass of water in one mole of the hydrate by the molar mass of the hydrate and multiplying by 100. Moles Lab Activity 4.
The quality of the crystals greatly depends on the quality of the copper sulfate compound used. 1 PubChem release 2019. Use the data table below to answer the following.
Copper Sulfate Fine Crystal. Calcium aluminium zinc and leadII ions give white precipitate with a few drops of sodium hydroxide. Molar Mass 16.
Hold it over the flame with a test tube holder and heat it for a few minutes with the mouth of the tube slightly below the base of the tube. More often than not x is a whole number but keep in mind that there are some exceptions. Compound A has a molar mass of 20 gmol and compound B has a molar mass of 30 gmol.
Divide the moles of water by the moles. Academiaedu is a platform for academics to share research papers. Yes because the chemical factor 15921271 is just a bit greater than unity indicating that the mass of the product will slightly exceed that of the copper consumed.
Since the only component other than H2O and Cu 2 is the sulfate ion SO 4 2 we can now determine the complete formula of the hydrated copperII sulfate. Crystals of hydrated copperII sulfate consist of CuH 2 O 4 2 centers linked to SO 2 4 ions. When determining the formula mass for a hydrate the waters of hydration must be included.
A fifth water resides elsewhere in the framework but does not bind directly to copper. Strand Molar Relationships Topic Investigating stoichiometry. Try to avoid using powered copper sulfate as it does not produce very good results.
CopperII sulfate also known as copper sulphate are the inorganic compounds with the chemical formula Cu SO 4 H 2 O x where x can range from 0 to 5. Allow the test tube and its contents to cool. A Case Study Journal of Science Education and Technology 17 466-482.
SAFETY EXPERIMENTAL PROCEDURE. Older names for this compound include blue vitriol bluestone vitriol of copper and Roman vitriol. The acute and subacute toxicities of several CrIII and CrVI compounds chromium3 chloride chromium3 nitrate chromium3 sulfate chromium trioxide potassium dichromate were determined in NZC and CxO mice injected ipThe distal median lethal doses 10 days after treatment averaged 179 or - 18 X 10-6 g chromiumg body wt regardless of the oxidation state of the Cr.
To do that calculate the hydrates percent composition by using its molar mass and the molar mass of anhydrous copperII sulfate. Calculating the Formula of a Hydrate 1. What is the percent water in copperII sulfate pentahydrate CuSO 4 5 H 2 O.
If there is a difference between the mass of carbon and hydrogen versus. 123 gmole Molar mass of I2 253.

Percent Composition Practice Problem 3 Cuso4 5h2o Youtube
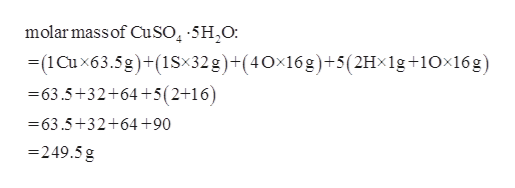
Answered Calculate The Molar Mass Of Cuso4 Bartleby
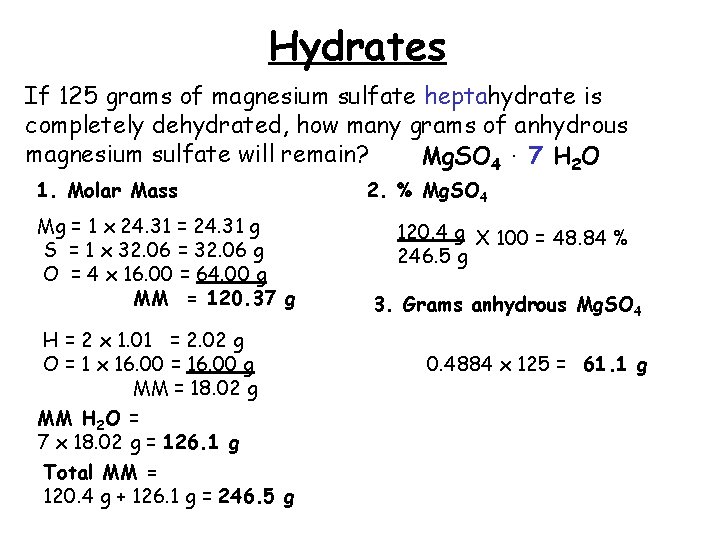
Percent Composition Empirical Formulas Molecular Formulas Percent Composition

Molar Mass Molecular Weight Of Cuso4 5h2o Copper Ii Sulfate Pentahydrate Youtube
Molar Relationships Ppt Download

Molar Mass Molecular Weight Of Cuso4 5h2o Copper Ii Sulfate Pentahydrate Youtube

Molar Mass Of Cuso4 5h2o Youtube

Molar Mass Molecular Weight Of Cuso4 5h2o Copper Ii Sulfate Pentahydrate Youtube
Molar Mass Molecular Weight Of Cuso4 Copper Ii Sulfate Youtube