Normally systemic acid-base balance is well regulated with arterial pH between 736 and 744. This quantity is the base excess in millimoles per liter and is considered negative if NaOH must be used acidosis and positive if HCl is needed alkalosis.
Acids and bases exist as conjugate acid-base pairsThe term conjugate comes from the Latin stems meaning joined together and refers to things that are joined particularly in pairs such as Brnsted acids and bases.

Sodium phosphate acid or base. Calcium Hydroxide and. ACIDBASE 15000 mmol of CO2. Polyphosphates are weak bases.
Sodium Phosphate and Calcium Chloride react to form Calcium Phosphate and Sodium Chloride. It makes use of the neutralization reaction that occurs between acids and bases and the knowledge of how acids and bases will react if their formulas are. Sodium lactate is an organic sodium salt having lactate as the counterion.
Intracellular pH is usually approximately 72. A salt is a combination of a base and an acid and is created when the positive ions of a base replace the positive hydrogen ions of an acid. Every time a Brnsted acid acts as an H -ion donor it forms a conjugate baseImagine a generic acid HA.
Test acid-base indicators with dilute HCl lemon juice vinegar ammonia solution dilute sodium hydroxide solution lime water tap water demineralized water. CID 612 Lactic acid Component Compounds. BUN may be increased as a result of renal dysfunction.
To determine whether phosphate supplementation started soon after birth in adequate quantity would prevent rickets in very low birth weight infants with prenatal deficiency of phosphate 40 neonates were given an initial dose of 50 mgday of phosphate administered as a mixture of 189 g of sodium phosphate dibasic disodium hydrogen phosphate and 82 g of sodium phosphate monobasic sodium. They may be weak acids that dissociate and change colour in alkaline solutions. When carbonic acid comes into contact with a strong base such as NaOH bicarbonate and water are formed.
It is a trivalent inorganic anion and a conjugate base of hydrogen phosphate. For instance chronic metabolic acidosis can be associated with decreased bone density nephrolithiasis muscle wasting and. And positively charged ions such as calcium or.
Phosphate sulfate lactate UC Unmeasured. Multiply Na excess by 07 and add to chloride if hypochloremic metabolic alkalosis or chronic resp acidosis if hyperchloremic metabolic acidosis or chronic resp alkalosis. 2 Al 6 HCl 3H 2 2 AlCl 3 Single Replacement 6.
It is composed of a group of salts containing the phosphate ion the dihydrogen phosphate ion or the hydrogen phosphate ion. Potassium deficit may occur especially if the client is receiving potassium. ChlorideSodium Correction 710 rule.
Add sodium hydroxide to. The bicarbonate is regulated in the blood by sodium as are the phosphate ions. Lactic acid sodium salt.
Clinical findings and history are also necessary to define the factors that may contribute to the development. A lone pair of electrons on an oxygen atom can be donated to a hydrogen ion proton or a metal ion in a typical Lewis acid-Lewis base interaction. ABGs may reflect metabolic acidosis.
Acid HCl or base sodium hydroxide NaOH in milli-moles required to return a liter of blood to pH 740. Using the stoichiometry of the reaction the unknown concentration can be determined. When sodium bicarbonate NaHCO 3 comes into contact with a strong acid such as HCl carbonic acid H 2 CO 3 which is a weak acid and NaCl are formed.
When this acid donates an H ion to water. This has profound significance in biology. Approach To ALL AcidBase Problems Dont get overwhelmed by all the numbers at once.
CID 5360545 Sodium CID 612 Lactic acid Dates. Phosphate is also called Phosphate ion or Orthophosphate. It has a role as a food preservative and a food acidity regulator.
An acid-base titration is a neutralization reaction performed in the lab to determine an unknown concentration of acid or base. Acid-base homeostasis and pH regulation are critical for both normal physiology and cell metabolism and function. When Na 2 HPO4 2- comes into contact with a strong acid such as HCl the base picks up a second hydrogen ion to form the weak acid Na 2 H 2 PO 4 and sodium chloride NaCl.
Na 2 CO 3 H 3 PO 4 Na 2 HPO 4 CO 2 H 2 O Na 2 HPO 4 NaOH Na 3 PO 4 H 2 O Uses Cleaning. Another option is to neutralize any base on the skin with a weak acid such as vinegar and then rinse with water. The moles of acid will equal the moles of the base at the equivalence point.
For instance adenosine triphosphate is about 25 protonated in aqueous solution at pH 7. PO 4 3- is a chemical derivative of phosphoric acid with a chemical name Phosphate. An acid-base titration is used to determine the unknown concentration of an acid or base by neutralizing it with an acid or base of known concentration.
Wilson and Green 1985. Replace potassium losses as indicated. So if you know one value you automatically know the other.
Acid-base and complexation properties. When Na 2 HPO4 2 the. Everyday processes like walking the digestion of food and the overall metabolism in your body produce a lot of acid as a byproduct.
Potassium metal and Chlorine gas combine to form 2 K Cl 2 2KCl Synthesis 5. Aluminum and Hydrochloric acid react to form Aluminum Chloride and Hydrogen gas. As an alternative some use the base deficit which has the opposite sign of the base excess so that as acidosis worsens and.
Acid-base indicators change colour in acidic or basic solutions. 2Na 3 PO 4 3 CaCl 2 6NaCl Ca 3 PO 4 2 Double replacement 4. Potassium deficit may occur with kidney dysfunction or diuretic therapy.
The body contains several important salts like sodium chloride potassium chloride calcium chloride calcium carbonate calcium phosphate and sodium phosphate. Evaluation of mixed acid-base abnormalities requires an understanding of the anion gap the relationship between the change in serum sodium and chloride concentration and the limits of compensation for the primary acid-base imbalances Saxton and Seldin 1986. Phosphates are found in the blood in two forms.
Sodium dihydrogen phosphate Na 2 H 2 PO 4 which is a weak acid and sodium monohydrogen phosphate Na 2 HPO4 2- which is a weak base. Trisodium phosphate was at. It is an organic sodium salt and a lactate.
Heres how to perform the calculation to find your unknown. Because of this youd be a giant walking. Stir the sodium hydroxide a little at a time into a large volume of water and then dilute the solution to make one liter.
Trisodium phosphate is produced by neutralization of phosphoric acid using sodium carbonate which produces disodium hydrogen phosphateThe disodium hydrogen phosphate is reacted with sodium hydroxide to form trisodium phosphate and water. Extracellular fluid shifts sodium and water restriction and renal function all affect serum sodium levels.

Acid And Base Iman Alajeyan Acid Base Theory Acids In Water Solutions Show Certain Properties They Taste Sour And Turn Litmus Paper Red They React Ppt Download

Base Data Used For Phosphoric Acid And Sodium Phosphate Download Scientific Diagram

Base Data Used For Phosphoric Acid And Sodium Phosphate Download Scientific Diagram
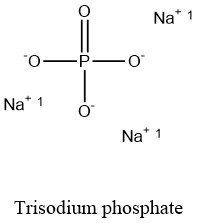
Sodium Phosphate Chemical Formula

Is Na3po4 Acidic Basic Or Neutral Dissolved In Water Youtube

Sodium Phosphate Monobasic Reagentplus 99 0 7558 80 7

Solved I Am Confused On How To Set Up The Problem 1 Also Chegg Com

Is Na3po4 Acidic Basic Or Neutral Dissolved In Water Youtube
