Give the formula for the following. E-mail to a friend.
Formula writing rules to write the correct chemical formulas for each compound.
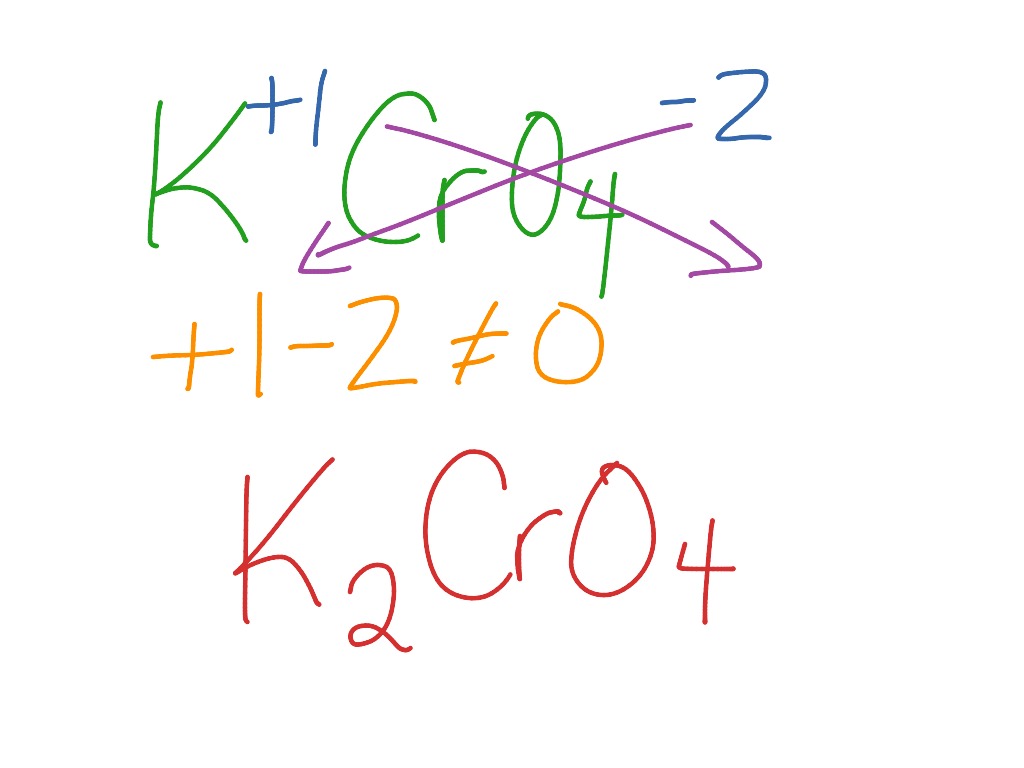
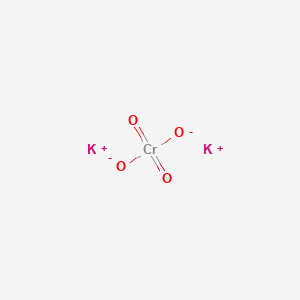
Formula for potassium chromate. This way students can see that the ions combine in whole number ratios in order to produce a neutral chemical species. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. KNO3 potassium nitrate 8.
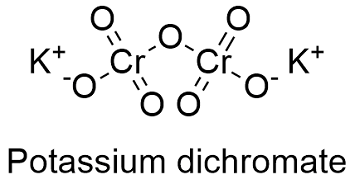
Give either the name or formula with the correct charge for each of the cations. It is an ionic compound with two potassium ions K and the negatively charged dichromate ion Cr2O7- in which two hexavalent chromium atoms with oxidation state 6 are each attached to three oxygen atoms as well as a bridging oxygen atom. Na2CrO4 sodium chromate 19.
H 3 O ferric. Among them is lópezite. The salt is popular in the laboratory because it is not.
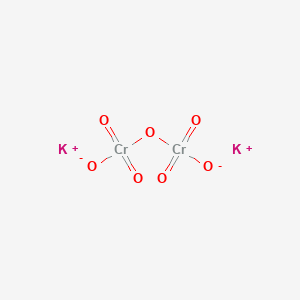
Potassium dichromate K 2 Cr 2 O 7 is a common inorganic chemical reagent most commonly used as an oxidizing agent in various laboratory and industrial applications. The chemical formula for potassium dichromate is K 2 Cr 2 O 7 and the molar mass is calculated to be 294185 gmol. You should complete this by Sunday.
Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound. LiCIO3 lithium chlorate 10. Sodium thiosulfate ____Na2S2O3_____ 3.
Its important to remember that common names are inaccurate and vary from one place and time to another. Give the formula for each compound. The chemical formula calculator also contains the names of a range of.
CdOH2 cadmium hydroxide 13. Ionic Compounds Summary Name the following compounds. Write the Formula Formula Unit for the following Compounds.
All that is left is to convert the yellow potassium chromateVI solution into orange potassium dichromateVI solution. NH42SO4 ammonium sulfate 20. Chromate containing minerals are rare.
This substance is used in the manufacture of dyes and in textile dyeing processes. An alloy of sodium and potassium NaK is used as a heat-transfer medium. Rare potassium chromate minerals and related compounds are found in the Atacama desert.
Use the stock form for the transition metals. Potassium chromate is highly corrosive and is a strong oxidizing agent. NiS nickel II sulfide Write the chemical formula for each of the following compounds.
Potassium chromate primarily affects the nose throat and lungs causing. If there is a common subscript such as 2 as in. MgOH2 magnesium hydroxide 9.
FeNO22 iron II nitrite 9. This reaction is also described further up the page. Cu2CO3 copper I carbonate 8.
Potassium carbonate K 2 CO 3 2. Many potassium salts are of utmost importance including the hydroxide nitrate carbonate chloride chlorate. C 2 O 4 2-Peroxide.
NO 2 barium. PbCO32 lead II carbonate 12. Notes about the Materials The list of ions was based on a dry lab from JA.
S 2 O 3 2-Carbonate. Which is also. Ionic Compounds Naming and Formula Writing.
It is a crystalline ionic solid with a very bright red-orange color. Chromate CrO 4 2-cyanide CN-dichromate Cr 2 O 7 2-dihydrogen phosphate H 2 PO 4 - or H 2 O 4 P-formate CHO 2-or HCOO-or CHOO-hydrogen sulfate or bisulfate HSO 4-hydrogen sulfite or bisulfite HSO 3-hydrogen phosphate HPO 4 2-hydroxide OH-hypochlorite ClO-nitrate NO 3-nitrite NO 2-oxalate C 2 O 4 2-perchlorate ClO 4-permanganate MnO 4-peroxide O. SnCN2 tin II cyanide.
B the chemical formula of the compound appears after the arrow. Chemical or scientific names are used to give an accurate description of a substances composition. Chemical Formula Nomenclature Practice.
31 cobalt III chromate Co2CrO43 32 ammonium oxide NH42O 33 potassium hydroxide KOH 34 lead IV sulfate PbSO42 35 silver cyanide AgCN 36 vanadium V nitride V3N5 37 strontium acetate SrC2H3O22 38 molybdenum sulfate MoSO43 39 platinum II. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions.
Each card has the name in both the old system and the stock system. NH42CrO4 ammonium chromate 17. As with all hexavalent chromium compounds it is acutely and chronically harmful to health.
In the event such products are used goggles should be worn at all times. In its solid form potassium chloride. 2 reduce it to.
Even so you rarely ask someone to pass the sodium chloride at the dinner table. Avoid all of these. Copy this to my account.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. You may remember that that is done by adding acid. At work request a material safety data sheet to help identify alternatives that are safe hence avoiding contact with material containing chromates.
ZnHCO32 zinc bicarbonate 15. This is described above if you have forgotten. Potassium chloride is an ionic salt featuring a bond between an alkali metal and a halogen.
Iron II chloride 18. Potassium chloride is characterized by a colourless crystalline appearance and an odourless smell. Identify the charges Mg.
Compound Name Type of Compound. Sodium sulfate Na 2 SO 4. Potassium Chromate is a yellowish crystalline inorganic compound that emits toxic chromium fumes upon heating.
BaClO42 barium perchlorate 11. Hg2NO22 mercury I nitrite 16. It is denoted by the chemical formula KCl and is made up of potassium cations and chloride anions in a 11 ratio.
Complete these in lab and on your own time for practice. If the subscript is a 1 it does not need to be written. Cr 2 K 2 0 7 the hexavalent form of.
SnSO4 tin II sulfate 14. The solution turns yellow as potassium chromateVI is formed. Parentheses and a subscript are not used unless more than one of a polyatomic ion is present in the formula unit eg calcium sulfate CaSO 4 not CaSO 4.
O 2 2--3 ions. Potassium is an essential constituent for plant growth and is found in most soils. Crocoite PbCrO 4 which can occur as spectacular long red crystals is the most commonly found chromate mineral.
Sodium chloride and potassium chloride. Sodium nitrite NaNO2 31. Cross the Charges Mg.
Sulfur dioxide ____SO2_____ 2. Write Formula Unit For the Below. K2Cr2O7 potassium dichromate 6.
KHCO3 potassium bicarbonate 7. AgNO3 silver nitrate 10. Determining the formula for Magnesium Fluoride.
Unfortunately there is a problem here. Potassium carbonate 2K CO 3 2- K 2 CO 3. Cr 2 O 7 2-Thiosulfate.
Beran - Laboratory Manual for Principles of General Chemistry.

Potassium Dichromate Bioxtra 99 5 7778 50 9
Potassium Chromate K2cro4 Pubchem
Potassium Dichromate K2cr2o7 Pubchem

How To Write The Formula For Potassium Chromate Youtube
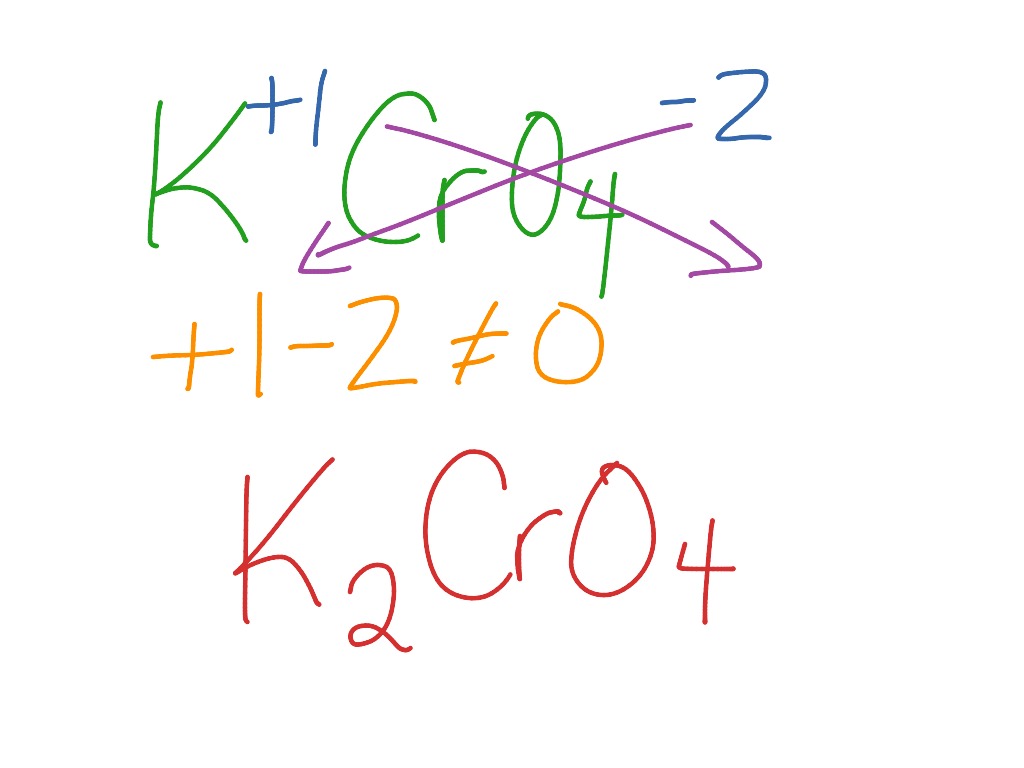
Formula For Potassium Chromate Science Chemistry Showme
Potassium Chromate Crk2o4 Chemspider

How To Write The Formula For Potassium Dichromate Youtube