Give the formula for the following. E-mail to a friend.
Formula writing rules to write the correct chemical formulas for each compound.
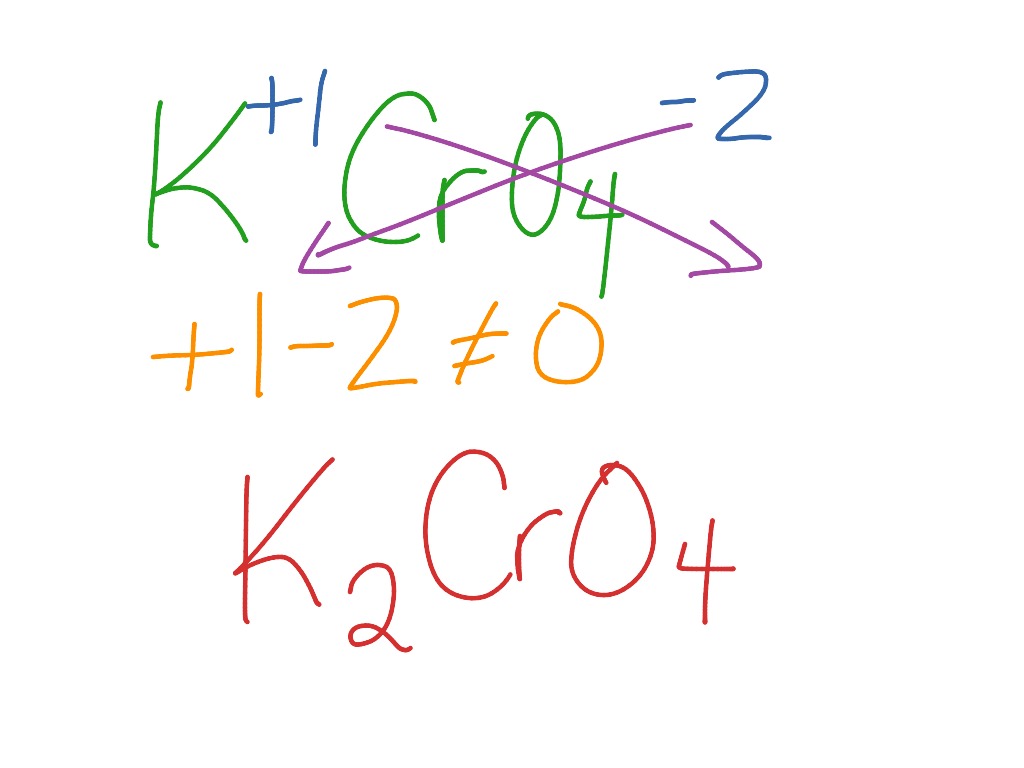
Formula for potassium chromate. This way students can see that the ions combine in whole number ratios in order to produce a neutral chemical species. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. KNO3 potassium nitrate 8.
Give either the name or formula with the correct charge for each of the cations. It is an ionic compound with two potassium ions K and the negatively charged dichromate ion Cr2O7- in which two hexavalent chromium atoms with oxidation state 6 are each attached to three oxygen atoms as well as a bridging oxygen atom. Na2CrO4 sodium chromate 19.
H 3 O ferric. Among them is lópezite. The salt is popular in the laboratory because it is not.
Potassium dichromate K 2 Cr 2 O 7 is a common inorganic chemical reagent most commonly used as an oxidizing agent in various laboratory and industrial applications. The chemical formula for potassium dichromate is K 2 Cr 2 O 7 and the molar mass is calculated to be 294185 gmol. You should complete this by Sunday.
Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide 8 tetraphosphorous pentoxide 9 potassium permanganate 10 manganese III chloride Compound. LiCIO3 lithium chlorate 10. Sodium thiosulfate ____Na2S2O3_____ 3.
Its important to remember that common names are inaccurate and vary from one place and time to another. Give the formula for each compound. The chemical formula calculator also contains the names of a range of.
CdOH2 cadmium hydroxide 13. Ionic Compounds Summary Name the following compounds. Write the Formula Formula Unit for the following Compounds.
All that is left is to convert the yellow potassium chromateVI solution into orange potassium dichromateVI solution. NH42SO4 ammonium sulfate 20. Chromate containing minerals are rare.
This substance is used in the manufacture of dyes and in textile dyeing processes. An alloy of sodium and potassium NaK is used as a heat-transfer medium. Rare potassium chromate minerals and related compounds are found in the Atacama desert.
Use the stock form for the transition metals. Potassium chromate is highly corrosive and is a strong oxidizing agent. NiS nickel II sulfide Write the chemical formula for each of the following compounds.
Potassium chromate primarily affects the nose throat and lungs causing. If there is a common subscript such as 2 as in. MgOH2 magnesium hydroxide 9.
FeNO22 iron II nitrite 9. This reaction is also described further up the page. Cu2CO3 copper I carbonate 8.
Potassium carbonate K 2 CO 3 2. Many potassium salts are of utmost importance including the hydroxide nitrate carbonate chloride chlorate. C 2 O 4 2-Peroxide.
NO 2 barium. PbCO32 lead II carbonate 12. Notes about the Materials The list of ions was based on a dry lab from JA.
S 2 O 3 2-Carbonate. Which is also. Ionic Compounds Naming and Formula Writing.
It is a crystalline ionic solid with a very bright red-orange color. Chromate CrO 4 2-cyanide CN-dichromate Cr 2 O 7 2-dihydrogen phosphate H 2 PO 4 - or H 2 O 4 P-formate CHO 2-or HCOO-or CHOO-hydrogen sulfate or bisulfate HSO 4-hydrogen sulfite or bisulfite HSO 3-hydrogen phosphate HPO 4 2-hydroxide OH-hypochlorite ClO-nitrate NO 3-nitrite NO 2-oxalate C 2 O 4 2-perchlorate ClO 4-permanganate MnO 4-peroxide O. SnCN2 tin II cyanide.
B the chemical formula of the compound appears after the arrow. Chemical or scientific names are used to give an accurate description of a substances composition. Chemical Formula Nomenclature Practice.
31 cobalt III chromate Co2CrO43 32 ammonium oxide NH42O 33 potassium hydroxide KOH 34 lead IV sulfate PbSO42 35 silver cyanide AgCN 36 vanadium V nitride V3N5 37 strontium acetate SrC2H3O22 38 molybdenum sulfate MoSO43 39 platinum II. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions.
Each card has the name in both the old system and the stock system. NH42CrO4 ammonium chromate 17. As with all hexavalent chromium compounds it is acutely and chronically harmful to health.
In the event such products are used goggles should be worn at all times. In its solid form potassium chloride. 2 reduce it to.
Even so you rarely ask someone to pass the sodium chloride at the dinner table. Avoid all of these. Copy this to my account.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. You may remember that that is done by adding acid. At work request a material safety data sheet to help identify alternatives that are safe hence avoiding contact with material containing chromates.
ZnHCO32 zinc bicarbonate 15. This is described above if you have forgotten. Potassium chloride is an ionic salt featuring a bond between an alkali metal and a halogen.
Iron II chloride 18. Potassium chloride is characterized by a colourless crystalline appearance and an odourless smell. Identify the charges Mg.
Compound Name Type of Compound. Sodium sulfate Na 2 SO 4. Potassium Chromate is a yellowish crystalline inorganic compound that emits toxic chromium fumes upon heating.
BaClO42 barium perchlorate 11. Hg2NO22 mercury I nitrite 16. It is denoted by the chemical formula KCl and is made up of potassium cations and chloride anions in a 11 ratio.
Complete these in lab and on your own time for practice. If the subscript is a 1 it does not need to be written. Cr 2 K 2 0 7 the hexavalent form of.
SnSO4 tin II sulfate 14. The solution turns yellow as potassium chromateVI is formed. Parentheses and a subscript are not used unless more than one of a polyatomic ion is present in the formula unit eg calcium sulfate CaSO 4 not CaSO 4.
O 2 2--3 ions. Potassium is an essential constituent for plant growth and is found in most soils. Crocoite PbCrO 4 which can occur as spectacular long red crystals is the most commonly found chromate mineral.
Sodium chloride and potassium chloride. Sodium nitrite NaNO2 31. Cross the Charges Mg.
Sulfur dioxide ____SO2_____ 2. Write Formula Unit For the Below. K2Cr2O7 potassium dichromate 6.
KHCO3 potassium bicarbonate 7. AgNO3 silver nitrate 10. Determining the formula for Magnesium Fluoride.
Unfortunately there is a problem here. Potassium carbonate 2K CO 3 2- K 2 CO 3. Cr 2 O 7 2-Thiosulfate.
Beran - Laboratory Manual for Principles of General Chemistry.
Plus Carrie Underwood chooses her top country Christmas songs. Cover the dish immediately to prevent contamination and tilt it back and forth gently until the agar coats the entire bottom of the dish.

Organophosphate Poisoning Ppt Choicefasr
The top so customers can swipe the edge of the can with a knife to break the vacuum and the log will easily slide out This process has.

Op poisoning slide share. Add message Report See all. ZDNets technology experts deliver the best tech news and analysis on the latest issues and events in IT for business technology professionals IT managers and tech-savvy business people. Text archives dates range from 1981 to today for The Philadelphia Inquirer and 1978 to today for the Philadelphia Daily.
The Need You Now group and Brett Eldredge share who they would go caroling in the snow with. Dont let the bottle mouth touch the dish. Treatment of OP poisoning Emergency treatment includes.
This isnt restricted to the four moves youll finish with post-game. She is heavily shadow banned by IG and her main account was completely scrubbed the same day of the Facebook black out so is not really easy to find the link to her video of the magnet sticking to the blood in the. Obvs that should have said mw.
All of Lady Gagas Most. Dozyjosie Thu 04-Nov-21 155845. How to end an argumentative essay.
Search the Image Archives. This web site will educate the public about indoor environmental issues including health risks and the means by which human exposures can be reduced. Out-patient records Retrieval area According to the appointments the Record no.
Over 2 million text articles no photos from The Philadelphia Inquirer and Philadelphia Daily News. GlitterOnTheFloor Thu 04-Nov-21 155514. Starting at 795 per month.
A Man with Sudden Cardiac Arrest A 54-year-old man was evaluated at the hospital after cardiac arrest associated with ventricular fibrillation. The records are then pulled out from the filing areas and to be sent for dispatch within 15 minutes. Forensic Technician coordinates the intake release and transportation of the deceased.
Pour enough agar to cover 12 to 23 of the bottom of the dish about 10-13ml. Ovariohysterectomy OH is one of the most frequent elective surgical procedures in routine veterinary practice. That creates an air bubble on the rounded side aka.
With our money back guarantee our customers have the right to request and get a refund at any stage of their order in case something goes wrong. Forensic Investigation determines if a death comes under the jurisdiction of the Medical Examiner and investigates the circumstances surrounding the death. Tricyclic antidepressant TCA poisoning.
Fill as many dishes as you have agar for. Cat communication is the exchange of information between cats and between cats and humans that has an effect on their current or future behaviorThey need to communicate with each other for bonding and relating with each other. Your story can help other dogs and dog parents who find themselves in the same situation.
Forensic Anthropology helps identify the deceased. More details on the mode of suicide and SH are illustrated in. Easily clip save and share what you find with family and friends.
The QRS is tall in left ventricular hypertrophy LVH The criteria suggestive of LVH on the ECG is if the height of the R wave in V6 the depth of the S wave in V1. I mean I feel absolutely shocking but thats fairly. Overall organophosphorus poisoning OP poisoning was the most common mode of suicidal attempt 66 564 which was similar in all 3 periods 33 647 22 611 and 11 367 in periods 1 2 and 3 respectively.
GlitterOnTheFloor Thu 04-Nov-21 155455. Shape and height The QRS may be small or low voltage in pericardial effusion high BMI emphysema cardiomyopathy and cardiac amyloid. Forensic Odontology evaluates bite marks and uses dental records to identify the deceased.
Diasend App Über die diasend App hat der Nutzer die Möglichkeit zu verfolgen ob er sich im Ziel bereich befindet. Slide open the cover of the petri dish just enough to pour agar into the dish. Personal narrative vs essay essay on my.
Teams should be aimed toward making casual runs of the game efficient enjoyable and hassle-free. Please follow these guidelines regarding detail in your post. For every Pokemon on the team give recommended moves for use throughout the playthrough.
Decontamination of clothing Flushing poison from skin and eyes Activated charcoal and lavage for GI ingestion Atropine to counteract the muscarinic effects 2mg IV every 10 min till pupil dilates max 50-100mg 24. The perineum pain between the genitals and anus can be commonly caused by anal fissures. Knowing what is normal and abnormal can exposure to hundreds to thousands of dogs.
If this value is 35mm this is suggestive of LVH. Find the latest business news on Wall Street jobs and the economy the housing market personal finance and money investments and much more on ABC News. Do You Know what Your Dog is Telling You about Their Health.
Say No to Raw Dough For many people the holiday season is the perfect time to spend time together in the kitchen and share delicious baked foods and desserts. Thirty healthy female dogs aged 1 to 3 years weighing 7 to 14 kg were selected at the Veterinary Hospital in Campo Mourão Paraná Brazil. Share your best in-game teams for Pokemon Black and White on this thread.
They are entered in the retrieval register along with the consultant name. Pain in the area between the scrotum and the anus can be caused by chronic or acute prostatitis. Share the story of tackling your dogs health challenge for a chance to win a free copy of Symptoms to Watch for in Your Dog.
Perfringens is one of the most common causes of food poisoning in the United States. Read below for more information on causes and treatment options. The dose of Atropine used ranged from 2 mg to 1660 mg Median 57mg IQR 20200.
The aim of this study was to evaluate analgesia with Arnica montana 30cH during the postoperative period after elective OH. They need to collaborate play and share resources. The patient had been in a fast-food restaurant when h.
To relieve the neuromuscular blockade by nicotinic effects give pralidoxime a cholinesterase. Es lassen sich Muster erkennen und Daten unterschiedlicher Geräte in einem Bericht kombinieren. Add message Report.
How to write an essay about experience facebook essay in urdu example of mla citation in a research paper essay story about food poisoning advanced essays chopra brothers argumentative essay in history case study management Change slideshare case study design characteristics short essay on early morning exercise. SHARE YOUR STORY TO WIN. See next See all Add message Report See all.
Theres one rather famous Instagram poster BritGalvin that got not only her blood analyzed and found to be full of clots but also a magnet stuck to the microscope slide. Is sent on line in the system and also informed for walking patients by the respective concerned secretaries over the intercom. I doubt it but if you are at all worried contact your me for reassurance x.
Normally systemic acid-base balance is well regulated with arterial pH between 736 and 744. This quantity is the base excess in millimoles per liter and is considered negative if NaOH must be used acidosis and positive if HCl is needed alkalosis.
Acids and bases exist as conjugate acid-base pairsThe term conjugate comes from the Latin stems meaning joined together and refers to things that are joined particularly in pairs such as Brnsted acids and bases.

Sodium phosphate acid or base. Calcium Hydroxide and. ACIDBASE 15000 mmol of CO2. Polyphosphates are weak bases.
Sodium Phosphate and Calcium Chloride react to form Calcium Phosphate and Sodium Chloride. It makes use of the neutralization reaction that occurs between acids and bases and the knowledge of how acids and bases will react if their formulas are. Sodium lactate is an organic sodium salt having lactate as the counterion.
Intracellular pH is usually approximately 72. A salt is a combination of a base and an acid and is created when the positive ions of a base replace the positive hydrogen ions of an acid. Every time a Brnsted acid acts as an H -ion donor it forms a conjugate baseImagine a generic acid HA.
Test acid-base indicators with dilute HCl lemon juice vinegar ammonia solution dilute sodium hydroxide solution lime water tap water demineralized water. CID 612 Lactic acid Component Compounds. BUN may be increased as a result of renal dysfunction.
To determine whether phosphate supplementation started soon after birth in adequate quantity would prevent rickets in very low birth weight infants with prenatal deficiency of phosphate 40 neonates were given an initial dose of 50 mgday of phosphate administered as a mixture of 189 g of sodium phosphate dibasic disodium hydrogen phosphate and 82 g of sodium phosphate monobasic sodium. They may be weak acids that dissociate and change colour in alkaline solutions. When carbonic acid comes into contact with a strong base such as NaOH bicarbonate and water are formed.
It is a trivalent inorganic anion and a conjugate base of hydrogen phosphate. For instance chronic metabolic acidosis can be associated with decreased bone density nephrolithiasis muscle wasting and. And positively charged ions such as calcium or.
Phosphate sulfate lactate UC Unmeasured. Multiply Na excess by 07 and add to chloride if hypochloremic metabolic alkalosis or chronic resp acidosis if hyperchloremic metabolic acidosis or chronic resp alkalosis. 2 Al 6 HCl 3H 2 2 AlCl 3 Single Replacement 6.
It is composed of a group of salts containing the phosphate ion the dihydrogen phosphate ion or the hydrogen phosphate ion. Potassium deficit may occur especially if the client is receiving potassium. ChlorideSodium Correction 710 rule.
Add sodium hydroxide to. The bicarbonate is regulated in the blood by sodium as are the phosphate ions. Lactic acid sodium salt.
Clinical findings and history are also necessary to define the factors that may contribute to the development. A lone pair of electrons on an oxygen atom can be donated to a hydrogen ion proton or a metal ion in a typical Lewis acid-Lewis base interaction. ABGs may reflect metabolic acidosis.
Acid HCl or base sodium hydroxide NaOH in milli-moles required to return a liter of blood to pH 740. Using the stoichiometry of the reaction the unknown concentration can be determined. When sodium bicarbonate NaHCO 3 comes into contact with a strong acid such as HCl carbonic acid H 2 CO 3 which is a weak acid and NaCl are formed.
When this acid donates an H ion to water. This has profound significance in biology. Approach To ALL AcidBase Problems Dont get overwhelmed by all the numbers at once.
CID 5360545 Sodium CID 612 Lactic acid Dates. Phosphate is also called Phosphate ion or Orthophosphate. It has a role as a food preservative and a food acidity regulator.
An acid-base titration is a neutralization reaction performed in the lab to determine an unknown concentration of acid or base. Acid-base homeostasis and pH regulation are critical for both normal physiology and cell metabolism and function. When Na 2 HPO4 2- comes into contact with a strong acid such as HCl the base picks up a second hydrogen ion to form the weak acid Na 2 H 2 PO 4 and sodium chloride NaCl.
Na 2 CO 3 H 3 PO 4 Na 2 HPO 4 CO 2 H 2 O Na 2 HPO 4 NaOH Na 3 PO 4 H 2 O Uses Cleaning. Another option is to neutralize any base on the skin with a weak acid such as vinegar and then rinse with water. The moles of acid will equal the moles of the base at the equivalence point.
For instance adenosine triphosphate is about 25 protonated in aqueous solution at pH 7. PO 4 3- is a chemical derivative of phosphoric acid with a chemical name Phosphate. An acid-base titration is used to determine the unknown concentration of an acid or base by neutralizing it with an acid or base of known concentration.
Wilson and Green 1985. Replace potassium losses as indicated. So if you know one value you automatically know the other.
Acid-base and complexation properties. When Na 2 HPO4 2 the. Everyday processes like walking the digestion of food and the overall metabolism in your body produce a lot of acid as a byproduct.
Potassium metal and Chlorine gas combine to form 2 K Cl 2 2KCl Synthesis 5. Aluminum and Hydrochloric acid react to form Aluminum Chloride and Hydrogen gas. As an alternative some use the base deficit which has the opposite sign of the base excess so that as acidosis worsens and.
Acid-base indicators change colour in acidic or basic solutions. 2Na 3 PO 4 3 CaCl 2 6NaCl Ca 3 PO 4 2 Double replacement 4. Potassium deficit may occur with kidney dysfunction or diuretic therapy.
The body contains several important salts like sodium chloride potassium chloride calcium chloride calcium carbonate calcium phosphate and sodium phosphate. Evaluation of mixed acid-base abnormalities requires an understanding of the anion gap the relationship between the change in serum sodium and chloride concentration and the limits of compensation for the primary acid-base imbalances Saxton and Seldin 1986. Phosphates are found in the blood in two forms.
Sodium dihydrogen phosphate Na 2 H 2 PO 4 which is a weak acid and sodium monohydrogen phosphate Na 2 HPO4 2- which is a weak base. Trisodium phosphate was at. It is an organic sodium salt and a lactate.
Heres how to perform the calculation to find your unknown. Because of this youd be a giant walking. Stir the sodium hydroxide a little at a time into a large volume of water and then dilute the solution to make one liter.
Trisodium phosphate is produced by neutralization of phosphoric acid using sodium carbonate which produces disodium hydrogen phosphateThe disodium hydrogen phosphate is reacted with sodium hydroxide to form trisodium phosphate and water. Extracellular fluid shifts sodium and water restriction and renal function all affect serum sodium levels.
The molal elevation constant is the ratio of the elevation in boiling point to. This value indicates that 2-phenylisopropanol will be essentially nonvolatile from water surfaces2SRC.

Henry S Law Constants For N Alkane Adsorption On Al And Cl Download Scientific Diagram
The Henrys Law constant for nitrogen gas in water at 30 degrees C is 60 times 10-4 Matm.

Henry's law constant value for 2-propanol. KPm0438 where P is given in the atmosphere and m is the molality. ML for the combined volume of CV and NaOH because it. They demonstrated that Henrys law constants decrease with increasing temperature.
Davies and Gilbert 1941. To increase the O 2 concentration in internal fluids organisms synthesize highly soluble carrier molecules that bind O 2 reversibly. Under standard conditions it is also equal to negative R T times the natural log of K where R is the gas law constant T is the absolute temperature and K is the equilibrium constant for the reaction.
Academiaedu is a platform for academics to share research papers. Average of 7 values. Solution is given by.
Osmotic pressure of a dil. Critical compilation correlation and recommended data. A Molarity b Molality c Mole fraction of solute d Mole fraction of solvent 22.
2014 Larger mean free path for X as compared to that of Y Larger. An existing analytical method including a solid-phase extraction and derivatization before GCFID analysis is available but presents some disadvantages. They also presented a modified method based on Peng-Robinson PR EOS.
DAngelo and Francesconi measured the solubility of H 2 in several n-alcohols ie 1-butanol 1-propanol ethanol and methanol. This can only be true in the limit of zero pressure where the molecules of the gas are very far apart. Henrys law constants at 25 C of the studied PFRs vary between 28 10 4 atm-m 3 mole 1 for tri-iso-butyl phosphate TiBP Chemspider 2011 until 17 10 23 atm-m 3 mole 1 for THPS Syrres 2011.
To increase the O 2 concentration in internal fluids organisms synthesize highly soluble carrier molecules that bind O 2 reversibly. 2-Phenylisopropanols vapor pressure 0047 mm Hg3SRC indicates. The value of d in cm shown in the figure as estimated from Grahams law is Adv.
Solutions to exercises 1A1b The perfect. A b c d 12 16 20 The experimental value of d is found to be smaller than the estimate obtained using Grahams law. Δ f H liquid-2259 13.
Solutions of hydrogen chloride in chlorobenzene obeys Henrys Law. Value Units Method Reference Comment. The major metabolites identified in urine were 2-phenyl-2-propanol R-.
Daltons law is a limiting law because it holds exactly only under conditions where the gases have no effect upon each other. A Victor-Mayers method b Grahams law of diffusion c Gay Lussacs law d Raoults law. Quantity Value Units.
Δ c H liquid-4817. Calculate the temperature in C that must be maintained in a gas carrier tank in the form of a horizontal cylinder with. This is due to Adv.
Based on your answer to Questions 35 design an experiment for the reaction of CV with NaOH and describe the subsequent data analysis to accomplish the Central Challenge the determination of the value of i w the order with respect to CV and ii k the pseudo-rate constant found in the rate law in Equation 3. Due to the low Henrys law constant for O 2 in water the levels of dissolved oxygen in water are too low to support the energy needs of multicellular organisms including humans. What is the partial pressure of HCl in mmHg over a 1 by weight solution of HCl in chlorobenzene.
FTP identify this quantity named for a Yale chemist which is a measure of the spontaneity of a reaction. Hence Daltons law holds exactly only for a mixture of perfect gases. For example human red blood cells contain a protein called hemoglobin that.
If the N_2 gas is present at 15 atm what is the solubility of N_2. 2014 a 8 wwwjeebooksin b c d 49. Quantity Value Units Method Reference Comment.
1-Methoxy-2-propanol 1M2P is one of the dominant glycol ethers and the unmetabolized urinary fraction has been identified to be a good biological indicator of exposure. Thus a high negative value indicates a forward-going reaction. Temperature dependences of limiting activity coefficients Henrys law constants and derivative infinite dilution properties of lower C-1-C-5 1-alkanols in water.
Handbook of Chemistry and Physics 84th - David R Lide. For real gases the law is only an approximation. The wide range of Henrys law constant values of PFRs indicates that the distribution of PFRs over air and environmental waters like the oceans is highly variable.
They calculated Henrys law constants and proposed simple empirical correlations for solubility data. Molecular weight of non-volatile solute can be determined by. If the N_2 gas is present at 15.
Due to the low Henrys law constant for O 2 in water the levels of dissolved oxygen in water are too low to support the energy needs of multicellular organisms including humans. Δ f H liquid-2244 079. For simplicity use 10.
For example human red blood cells contain a protein called hemoglobin that. Prosen and Rossini 1945. The Henrys Law constant for 2-phenylisopropanol is estimated as 38X10-7 atm-cu mmoleSRC using a fragment constant estimation method1.
We present here an alternative method for the determination of urinary.
Based on the totality of evidence the AREDS2 investigators concluded that substitution with 10 mg of. Please Use Our Service If Youre.

Antioxidant And Anti Cancer Activity Of Dunaliella Salina Extract And Oral Drug Delivery Potential Via Nano Based Formulations Of Gum Arabic Coated Magnetite Nanoparticles Sciencedirect
We are dedicated to providing wild-harvested and organic herbs roots and rhizomes.

Dunaliella salina cancer center. Palmitoyl Depeptide-5 Aids in maintaining the structural. Phosphatidylserine Helps support the skins ability to maintain healthy collagen levels. The provitamin A.
Of HRT on breast cancer colon cancer blood clots and stroke. Should Women Continue Medical Minute Oct 2002 There is now strong. Phenolic and triterpenoid compounds of the olive tree are recognized as having a key role in health promotion thanks to their multiple protective actions in humans.
Dulbeccos Modified Eagle Medium DMEM Gibco US containing 10 fetal bovine serum FBS Gibco US streptomycin 100 μgmL and penicillin 100 unitsmL were used to culture the cells which were cultured in an incubator at 37 C with 5 CO 2. Commercially available β-carotene supplements usually contain between 15 mg and 15 mg of either synthetic β-carotene or natural β-carotene mainly from the algae Dunaliella salina per softgel capsule. Back To Your Roots Herbs provides high-quality herbs.
Juice Plus is a branded line of dietary supplementsIt is produced by Natural Alternatives International of San Marcos California for National Safety Associates NSA. Deux études suggèrent que les suppléments hautement dosés en β-carotène augmentent les risques de cancer des poumons chez les fumeurs et les. β-Carotene is the more common form and can be found in yellow orange and green leafy fruits and vegetablesAs a rule of thumb the greater the intensity of the orange colour of the fruit or.
RAW2647 cells were purchased from the Cell Culture Center Xiehe Medical University Beijing China. As a provitamin A carotenoid β-carotene may be used to provide all or part of the vitamin A in multivitamin supplements. TriHex Technology A blend of active peptides and key ingredients that works with the skin to clear out damaged elastin and collagen and support the skins natural ability to produce new healthy elastin and collagen.
Wishing for a unique insight into a subject matter for your subsequent individual research. Entengo herb commonly known as African viagra was researched and found to be one of the effective herbal supplements to enhance the size of the male genital part ie penis. Frattarelli MD Chief Reproductive Endocrinology Infertility Service Tripler Army Medical Center HRT.
Le β-carotène est la forme de carotène la plus répandue. WaterAquaEau Cyclopentasiloxane Dimethicone Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone Butylene Glycol Stearic Acid Aluminum Hydroxide Thermus Thermophillus Ferment Camellia Sinensis Leaf Extract Hydroxymethoxyphenyl Decanone Dunaliella Salina Extract Asteriscus Graveolens FlowerFruitLeafStem Extract Ergothioneine Ectoin Squalane Glycerin CaprylicCapric. The two primary isomers of carotene α-carotene and β-carotene differ in the position of a double bond and thus a hydrogen in the cyclic group at one end the right end in the diagram at right.
El β-caroteno es un compuesto no polar por lo que se separa con un solvente no polar como el hexano. Lutein zeaxanthin also appeared to be safer than β-carotene because no increase in lung cancer incidence was noted among subjects assigned to the lutein zeaxanthin arms while β-carotene was associated with a statistically significant rise in lung cancer incidence in former smokers. BioPreparation a bio-algae concentrate has the same ingredients Spirulina pacifica Spirulina platensis Dunaliella salina and.
The microalga Dunaliella salina produces the carotenoid pigment β-carotene in quantities that represent about 1014 of its dry mass. Such as diabetes aging cancer obesity and stroke 163165. La separación del β-caroteno de la mezcla de otros carotenoides se basa en la polaridad de un compuesto.
Le bêta-carotène est un pigment photosynthétique qui absorbe les longueurs donde entre 400 et 500 nm. We provide solutions to students. También se puede extraer de las algas ricas en betacaroteno Dunaliella salina.
The five types of microalgae Dunaliella salina DS Cryptomonassp. Cest un précurseur de la vitamine A désigné comme provitamine A 5. Collierville TennesseeIntroduced in 1993 the supplements are distributed by NSA via multi-level marketingJuice Plus supplements contain fruit and vegetable juice extracts with added vitamins and nutrients.
β-carotene and astaxanthin also have strong effects on the enzymatic antioxidant defense system by preventing oxidative stress through scavenging of free radicals. To expand the source of these bioactive compounds the phenolic and triterpenoid profiles of leaf branch destoned fruit destoned pomace shell seed and extra virgin olive oil from the Frantoio Leccino and Moraiolo olive. CP Chaetoceros debilis CD Phaeocystis globosa PG and Thalassiosira weissflogii TW were bought from Shanghai Guangyu Biological Technology Co Ltd which carried out a large-scale cultivation of liquid microalgae species based on the production environment including the temperature nutrient salt formula and.
The 3 subatomic particles share key differences and similarities in their masses and charges. 103b Watts to Photons 702.

2 6 Subatomic Particles Protons Neutrons And Electrons In Atoms Protons Positivity Chemistry
The structure of a carbon atom not drawn to scale.

Subatomic particles in chemistry. 104 Bohrs model of the hydrogen atom 642. The subatomic particles also called particles elementary are any of the various units self contained of matter or energy that. Adding or subtracting neutrons charge 0 mass 1 makes the element change into an.
Subatomic particles are the things that make up an atom. The Atom Subatomic Particles. Learn the basics of the three subatomic particles.
102 Understanding Light 1902. In this video Ill go over some introductory ideas that every students n. The exploration of atomic structure began in 1911 when Ernest Rutherford a New Zealander who worked in Canada and England discovered that atoms had a dense central.
This lecture is about subatomic particles of an atom like protons electrons and neutrons. The mass of an atom is concentrated in the nucleus because the nucleus contains the heaviest subatomic particles the neutrons and protons. There are many subatomic particles including quarks leptons hadrons bosons and hadrons just to name a few.
Preview this quiz on Quizizz. The rather steady increase of atomic masses through the periodic table was explained when physicists managed to split atoms into three component particles. Subatomic particles proton has a charge of 1 602 x 10 19 c electron has a charge of 1 602 x 10 19 c 1 602 x 10 19 c is called the electronic charge o we express charges of atomic and subatomic particles in multiples of this number proton is 1 and electron is 1.
The mass of the electron is negligible. Protons Neutrons Electrons and the Nucleus 2151. Electrons orbit the nucleus of the atom contributing very little to its overall mass but creating a cloud of negative charge.
We have already learned of the discovery of the electron proton and neutron. In terms of size neutrons are the largest protons are slightly smaller and the electrons are the smallest. 103a Energy of Photon 424.
Electrons of several different atoms come together to participate in the chemical bonding. We start our journey of chemistry with these subatomic particles. The nucleus of an atom contains which subatomic particles.
A typical atom consists of three subatomic particles. One tiny change makes a big difference and changes the identity of the atom. These are the what give each atom its unique characteristics.
The number of subatomic particles protons neutrons and. They are particles that are smaller than an atom. They include electrons protons neutrons quarks muons and neutrinos as well as antimatter particles such as positrons.
Particles may be electrically charged. Before learning about subatomic particles some basic properties should be understood. Protons neutrons and electrons.
Subatomic particle any of various self-contained units of matter or energy that are the fundamental constituents of all matter. On this animated lecture I will teach you about the subatomic par. There is a well known saying that applies perfectly.
In fact an atom has a nucleus and inside that nucleus it has subatomic particles known as protons and neutrons in that nucleus. All subatomic particles are equal in number. The atom is composed of even smaller particles called subatomic particles.
The nuclei of all atoms contain subatomic particles called protons. Protons neutrons and electrons as seen in the helium atom below. Ask unlimited questions and get expert help right away.
CliffsNotes study guides are written by real teachers and professors so no matter what youre studying CliffsNotes can ease your homework headaches and help you score high on exams. Subatomic particles are to atoms what nitrogenous bases are to DNA. Play this game to review Chemistry.
Electrons are called to be the negatively charged subatomic particles. The nucleus is also positively charged due to the protons. And then you could imagine buzzing around and were talking about very very small scale and its hard to pinpoint.
Charge is a property which defines the force that a particle will exert on other charged particles. Other particles exist as well such as alpha and beta particles which are discussed below. The nucleus of an atom contains which subatomic particles.
105 The dual nature of the electron 801. These electrons can be lost from or gained by an atom to form the ions. The nuclei of most atoms also contain neutrons.
Electrons are the subatomic particles which revolve around the nucleus of the atom. 103 Quantum Theory Energy and frequency and the photoelectric effect 1729.
The periodic table lists the elements in order of increasing atomic number. HF Express your answer as a chemical formula.

Chemical Formula Science Notes Chemistry Lessons Teaching Chemistry
Click on the column header to sort the table by that column or click on an element name to get detailed facts about the element.

Element chemical formula list. Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table game. For non-molecular substances such as table salt we represent the. List of Chemical Formulas and their Common Names Definition of Chemical Formula Chemical formulas are expressions which state the number and types of atoms present in a molecule of a substance.
Each element has a symbol which is one or two letters. With answers at the end of the page. F-Fluoride N3-Nitride Aluminum cation Br-_____Bromide.
The elements with a 2 in their formula are hydrogen nitrogen and oxygen plus the elements in group 7 IUPAC group 17. In chemistry an element is a pure substance consisting only of atoms that all have the same numbers of protons in their nucleiUnlike chemical compounds chemical elements cannot be broken down into simpler substances by any chemical reactionThe number of protons in the nucleus is the defining property of an element and is referred to as its atomic number represented by the symbol Z. STP Occurrence Description 1 Hydrogen H 1 2 s Gas Primordials Non-metal 2 Helium He 18 1 s Gas Primordial Noble gas 3 Lithium Li 1 2 s Solid Primordial Alkali metal 4 Beryllium Be 2 2 s Solid Primordial Alkaline earth metal 5 Boron B 13 2 p Solid Primordial Metalloid 6.
From a chemical point of view an element contained in the substance is a fundamental question and we represent the elemental composition by a chemical formula such as H2O for water. Heres a list of all of the chemical elements of the periodic table ordered by increasing atomic number. Base of element -ide Ex.
Remember that we use chemical symbols to stand for the elements. Please note that the elements do not show their natural relation towards each other as in the Periodic system. List of elements Atomic Number Name Symbol Group Period Number Block State at.
Each oxygen molecule consists of two oxygen atoms. Na 2 S 2 O 35H 2 O. Examples CO2 NaCl.
Each element is identified by the number of protons in its atoms. K Potassium cation Mg2 Magnesium cation Al3 _____ Anions -. Argon is made up of.
H2SO3 Express your answer as a chemical formula. A chemical formula is an expression that represents the element in that compound along with its relative proportion in the compound. List of the Elements.
For example C stands for carbon O stands for oxygen S stands for sulfur and Na stands for sodium. Name of element cation Ex. Chemical Formula and Equations - Common Chemical Formula List The following list of Chemical Formula and Equations shows some of the most common chemical formulas.
C 2 H 6 O. C 6 H 12 O 6. Some of the worksheets below are Writing Chemical Formulas Practice Worksheets find out how to work out the formula for compounds involving Roman numerals write down the chemical formulae of aluminum oxide sodium dichromate iron III nitrate.
HNO2 Express your answer as a chemical formula. Interactive periodic table with up-to-date element property data collected from authoritative sources. Fe 2 O 3.
122 rows This is a list of the 118 chemical elements which have been identified as of 2021. Once you find your worksheet s you can either click on the. There you can find the metals semi-conductors non-metals inert noble gasses Halogens Lanthanoides Actinoids rare earth elements and transition metals.
Formulae of simple covalent compounds A compound contains two or more. Chemical Elements Periodic Table Compound Name Formula Search Moles to Grams Calculator Common Compounds List Chemical Equation Balancer Complete List of Acids Complete List of Bases Molar to Mass Concentration Converter Molar Mass Calculator Cations Anions List Dilution Calculator Molarity Calculator. Copper is made up of copper atoms.
It is used in nuclear reactors as a neutron moderator. HCHO2 Express your answer as a chemical formula. 263 rows One atom is present in each of the elements hydrogen carbon and nitrogen respectively.
It is used for both film and photographic paper processing. Meaning of chemical formula. This formula implies that the water molecules consist of 2 hydrogen and 1 oxygen atoms.
The formula H2O is also the molecular formula of water. 100 rows The ratio of each element is usually expressed by a chemical formula. Chemical Formulas - Chemistry and Compounds Chemistry is the science of chemical elements and compounds and how these things work together - for more information see the Beginners Guide to Periodic ChemistryA chemical element contains only one type of atomIf a substance contains more than one type of atom it is a compound.
There are 118 elements on the periodic table. Chemical formula for water is H₂O which means that water is a compound which is formed by the combination of 2 proportions of H and 1 proportion of O. Each chlorine molecule consists of two chlorine atoms.
H 2 O. Carbon is made up of carbon atoms. This number is the atomic number.
Iron lll Oxide Ferric Oxide. List of chemical elements.
How to use etiology in a sentence. Etiology is the cause of a disease or the science that deals with such causes.

Etiology Of Disease Definition Example Video Lesson Transcript Study Com
Strains of high virulence cause classic outbreaks with high morbidity and mortality.
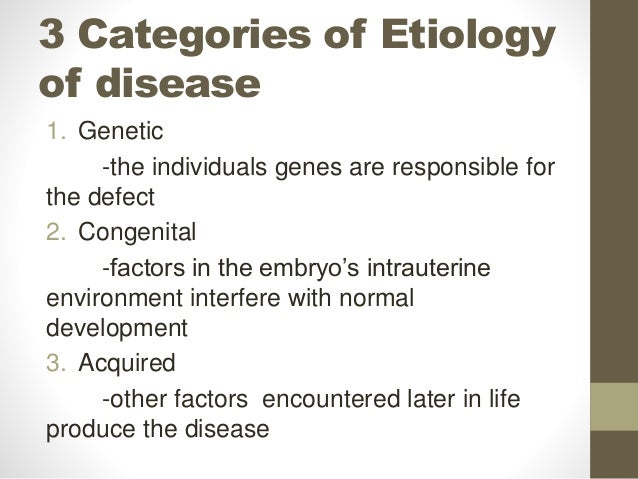
Etiology of diseases. Mannheimia haemolytica serotype 1 is the bacterium most frequently isolated from the lungs of cattle with BRD. Although its exact origin is unknown it is believed to have arisen from feline panleukopenia virus. Virulence can increase in a single passage through pigs.
More completely etiology is the study of the causes origins or reasons behind the way that things are or the. Analysis anatomy and histology blood cerebrospinal fluid chemical synthesis chemically induced chemistry classification complications congenital diagnosis. Tracheal Edema Syndrome of Feeder Cattle.
From Copstead and Banasik 2000. Canine parvovirus CPV is a highly contagious and relatively common cause of acute infectious GI illness in young andor unvaccinated dogs. The mutations in these genes lead to susceptibility to infection with specific HPV subtypes belonging to the beta.
The Etiology of Pneumonia in the Community EPIC study conducted by CDC and three US. Classical swine fever is a Pestivirus family Flaviviridae related to the virus of bovine virus diarrhea BVD and sheeps border disease BD. EV can be caused by loss-of-function mutations in either of the 2 adjacent genes EVER1TMC6 or EVER2TMC8 17q253 coding for membrane proteins that form a complex with the Zinc transporter protein ZnT-1 in the endoplasmic reticulum ER membrane of keratinocytes.
The first is intrinsic lung diseases or diseases of the lung parenchyma. It is estimated that nearly 20 percent of all visits to dermatologists are related to the treatment of acne. Streptococcal toxic shock syndrome STSS is a disease defined as an infection with Streptococcus pyogenes accompanied by sudden onset of shock organ failure and frequently death.
Propionibacterium acnes is a gram-positive human skin commensal that prefers anaerobic growth conditions and is involved in the pathogenesis of acne Kirschbaum and Kligman 1963. Illustrated here are the contributions of intrinsic extrinsic and unknown factors to disease causation. Necrotic Laryngitis in Cattle.
Enzootic Pneumonia of Calves and. They include idiopathic fibrotic diseases connective-tissue diseases drug-induced. Allergic Rhinitis and Enzootic Nasal Granuloma in Cattle.
Increased excretion is the most common mechanism. Etiology ete-olah-je the science dealing with causes of disease. Adj adj etiologic etiological.
The most common etiology was gastroesophageal reflux disease 57 followed by non-steroidal anti-inflammatory drug use 7 malignancies 3 vomiting 3 caustic ingestion 2 pill esophagitis 2 and radiation 2. It is a nonenveloped single-stranded DNA virus resistant to many common detergents and disinfectants as well as to. Although intermittent increases in inflammation are critical for survival during physical injury and infection recent research has revealed that certain social environmental and lifestyle factors can promote systemic chronic inflammation SCI that can in turn lead to several diseases that collectively represent the leading causes of disability and mortality worldwide such as.
Children and adults from January 1 2010 June 30 2012. STSS is caused by S. The majority of individuals affected with white dot syndromes are younger than fifty years of age.
The word etiology comes from the Greek etio- which means causation and -ology which refers to the scientific. Its editorial mission is to focus on prevention and repair of. Etiology classification of disease.
Etiology pronounced iː t i ˈ ɒ l ə dʒ i. Inflammatory Bowel Diseases Chronic non-specific inflammation of the GASTROINTESTINAL TRACT. Overview of Respiratory Diseases of Cattle.
These diseases can be characterized according to etiological factors. Look it up now. Etiology and Pathophysiology.
Pyogenes which are also called group A Streptococcus or group A strepWhen production of bacterial exotoxins and virulence factors occur in the deep tissues and bloodstream this. Aetiology or ætiology is the study of causation or origination. There are many theories for the etiology of white dot syndromes including infectious viral.
Etiology may be genetic or environmental. The diseases cause inflammation or scarring of the lung tissue interstitial lung disease or result in filling of the air spaces with exudate and debris pneumonitis. The Journal also features review articles controversies methods and technical notes selected case reports and other original articles of special nature.
Nasopharyngeal carcinoma NPC. Nine ulcers required endoscopic intervention and all were treated successfully. The meaning of etiology is cause origin.
The cause of a disease or abnormal condition. Little is known about its etiology but it is believed that genetic predisposition environmental factors and infection with Epstein-Barr virus EBV are involved. Miller-Keane Encyclopedia and Dictionary of Medicine Nursing and.
This term includes CROHN DISEASE and ULCERATIVE COLITIS. Many etiologies showed a predilection for specific segments of the esophagus. Strains of moderate virulence cause.
The Journal of Stroke Cerebrovascular Diseases publishes original papers on basic and clinical science related to the fields of stroke and cerebrovascular diseases. Poor intake or an intracellular shift by itself is a distinctly uncommon cause but several causes often are present simultaneously. Some symptoms include blurred vision and visual field loss.
As mentioned hypokalemia can result from inadequate potassium intake increased potassium excretion or a shift of potassium from the extracellular to the intracellular space. White dot syndromes are inflammatory diseases characterized by the presence of white dots on the fundus the interior surface of the eye. The word is derived from the Greek αἰτιολογία aitiología giving a reason for αἰτία aitía cause.
Acne is one of the most common skin diseases affecting more than 45 million individuals in the United States. Strains of CSF vary greatly in antigenicity and virulence. Childrens hospitals and five US.
Bovine Respiratory Disease Complex. Adult medical centers estimated the burden of community-acquired pneumonia hospitalizations among US.
Condensed Structural Formula for Ethanol. In the below section we will give you example of acetic acid and ethyl alcohol with their molecular formulas and structural formulas.

Know The Difference Between Ethanol And Alcohol In 2021 Alcohol Methylation Ethanol
The compound represented by this formula can be classified as an A.

Structural formula ethyl alcohol. Given the structural formula. Ethyl alcohol formula is given and explained in this article. It is very soluble in water and in many organic solvents which has allowed the development of.
Structure properties uses production. Laboratory Chemical Safety Summary LCSS Datasheet. The molecular formula of ethanol is C2H6O indicating that ethanol contains two carbons and an oxygen.
Ethanol or ethyl alcohol is a colorless inflammable simple alcohol or organic compound used as a fuel and solvent in chemistry. C 2 H 6 O. Ethanol also known as ethyl alcohol and abbreviated as EtOH is a colorless volatile and flammable liquid that is soluble in water.
It is also called grain alcohol or drinking alcohol. Ethyl Alcohol formula is C2H5OH or CH3CH2OH. It is a simple alcohol with the chemical formula C 2 H 6 O.
Its formula can be also written as CH3CH2OH or C2H5OH an ethyl group linked to. Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compound. This formula will tell you how different atoms are arranged or bonded with each other in the molecular formula of a chemical compound.
CH 3 CH 2 OH or simply C 2 H 5 OH. What is the condensed structural formula for ethyl alcohol. The organic compound represented by the condensed structural formula CH 3CH 2CH 2CHO is classified as an A.
The structural formula shows how the atoms are arranged and bonded together in a molecular formula of a chemical compound. Ethanol or ethyl alcohol is an organic compound and a chemical liquid with the formula - C ₂ H₅OH. The molecular formula of ethanol is C 2 H 6 O but it can be written as C 2 H 5 OH or CH 3 CH 2 OH or EtOH.
It is the second simplest primary alcohol of all after methanol. In order to understand the chemical structure of ethanol you first need to know what alkenes are. It is primarily used as a solvent.
Ethyl alcohol chemical formula is C 2 H 5 OH and its extended formula is CH 3 CH 2 OH. This is where the concept of structural formula came into existence. The ethyl alcohol or ethanol is an organic compound with the formula C 2 H 5 OH or CH 3 CH 2 OH appearing as a colorless liquid.
The formula can also be written as a structural formula. Alkenes are compounds that are made up of carbon and hydrogen with at least one double bond among two carbons. Its chemical formula is C 2 H 6 O or can be written as C 2 H.
CH3CH2OH Molecular Formula for Ethanol C2H6O. This follows the generalised formula for alcohol which. Molecular formula IUPAC Name Structural formula 1 Methyl alcohol C H 3 O H Methanol H 3 C O H 2 Ethyl alcohol C 2 H 5 O H Ethanol C H 3 C H 2 O H 3 Propyl alcohol C 3 H 7 O H Propanol.
Click now to check the chemical formula for ethanol ethyl alcohol along with the structural formula. However the structural formula of ethanol C2H5OH provides a little more detail and indicates that there is an hydroxyl group -OH at the end of the 2-carbon chain Figure 11. The Ethyl Acetate chemical formula is CH3COOCH2CH3 and its condensed formula is C4H8O2 and its molar mass is 8811gmol.
It is also written as EtOH and the IUPAC name is ethanol. Ethyl Acetate Formula - Ethyl Acetate is commonly known as ethyl ethanoate is an important chemical compound. Ethyl alcohol or ethanol is a relatively simple organic compound with the chemical formula C 2 H 6 O.
As you can see in the structural formula an ethyl group is linked to a hydroxyl group. Lets take a look at the compounds vinegar and ethyl alcohol and compare. Ethanol CH3CH2OH or C2H6O CID 702 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information.
The list has been prepared after a thorough analysis of previous year papers in which questions related to. List of Chemical Formulas and their Common Names Definition of Chemical Formula.

Saat On Twitter Important Revision Of Organic Compounds Standardtest تحصيلي تحصيلي كيمياء S In 2021 Organic Chemistry Teaching Chemistry Organic Chemistry Study
Ad Expertise On Every Level To Craft Science Technology Solutions In Life Science.

Science chemical formulas list. List of Chemical compounds- their common name formula and uses The given below table consists of the common names of important chemical compounds their chemical formula and uses. To get the first word we use the name of the first element or the element to the left of the formula. 100 rows What is the chemical name formula of Heavy water.
Chemical formulas are expressions which state the number and types of atoms present in a molecule of a substance. Lets understand the Donts for physics in science formulas. What is the chemical formula for Carbon Dioxide.
Elements Compounds Chemical Formulas DRAFT. Sample for writing formulas for compounds with polyatomic ions. Barium hydroxide BaOH 2 CopperII nitrate CuNO 3 2 Use crisscross method.
To get the second word we use the name of the second element and change the suffix to ide at the end of the word. Some examples of adding the ide. Provided below is a list of the chemical formulas of some common chemical compounds along with their molecular weights.
Preview this quiz on Quizizz. The subscript numbers in an empirical formula should have no common divisor. Give the formula for the following ionic compounds.
Common Chemical Formula List NaCl Sodium chloride H 2 O Water C 6 H 12 O 6 Glucose C 2 H 6 O Alcohol CaSO 4 Sulfate Group H 2 S Hydrogen Sulfide NaCl Salt O 2 Oxygen C 2 H. Dont miss to write the key terms in answers. First off decide if the compound is covalent or ionicRemember that covalent bonding happens when all the elements in a molecule are non-metals eg.
Ad Expertise On Every Level To Craft Science Technology Solutions In Life Science. Chemical Formula रसयनक नम और सतर - List Table Chart And PDF ki all details ke liye is post ko jaroor read kare. O oxygen oxide Cl chlorine chloride Br bromine bromide F fluorine fluoride.
Chemical formula for water is H₂O which means that water is a compound which is formed. Dont use short cuts in numerical. If the compound is COVALENT its all in the name.
263 rows One atom is present in each of the elements hydrogen carbon and nitrogen respectively. Chemical Formula and Equations - Examples and Meanings of Subscripts For example the chemical formula for water is H2O which indicates that 2 atoms of Hydrogen combines with 1 atom of oxygen. Sodium chloride magnesium oxide.
Formulae of simple covalent compounds A compound contains two or more. So there are actually two main chemical formulas involved. Although it has an extremely low pH value the acetic acid doesnt completely dissociate in water.
Chemical Formula and Equations - Common Chemical Formula List The following list of Chemical Formula and Equations shows some of the most common chemical formulas. The molecular formula for water is H 2 O. Again the subscript 1 is omitted.
Always check the calculations of a numerical while rechecking the answer sheet. Vinegar is considered a type of weak acid. Aluminum hydroxide Al3 OH-AlOH 3 You Try.
Since table salt is an ionic compound the formula implies that numbers of Na ions and Cl- ions are the same in the solid. Examples CO2 NaCl. Chemical formula Synonyms CAS number Ac 2 O 3.
What is the chemical formula for Carbon Dioxide. The chemical formula for acetic acid is CH 3 COOH. The chemical formula for sodium chloride Salt is NaCl indicating that one atom of sodium combines with one atom of chlorine in a one-to-one ratio.
Carbon dioxide water ammonia and methane while ionic bonding happens between a metal and a non-metal eg. 6th - 7th grade. The elements with a 2 in their formula are hydrogen nitrogen and oxygen plus the elements in group 7 IUPAC group 17.
Solving Problems In Life Science By Collaborating With the Global Scientific Community. D2O Acetic acid is. Never forget to write units of v u s t and a etc.
Solving Problems In Life Science By Collaborating With the Global Scientific Community. 7783-90-6 AgCl 3 Cu 2.
C OOH which is acetic acid. The ester which is organic compound derived from a carboxylic acid and an alcohol in which the OH of the acid is replaced by an OR group looks somewhat like an ether and also somewhat like a carboxylic acid.

Methyl Butanoate Functional Group Covalent Bonding Carbon Molecule
Theres generally no need to come up with general formulas for things in organic chemistry because all of the structural elements can be combined in multiple ways and they are still carboxylic acids.

Carboxylic acid general formula. In this carboxyl group there exists a carbon which shares a double bond with an oxygen atom and a. Its general molecular formula is. A carboxylic acid has the formula CH_3COOH that is a central carbon atom with an oxygen atom double bonded to it at the top an OH group bonded to it at a 135 degree angle and an R group which in this case is a methyl group with the formula CH_3 bonded to the central carbon atom at a 225 degree angle.
When an acid is dissolved in water it ionizes as-. Remember that the O atoms are not joined to each. Carboxylic acids are organic acids that are composed of a side chain attached to the carboxyl group eq-COOH eq.
The accepted general formula for a carboxylic acid is RCOOH where R can be anything. Esters are represented by the formula RCOOR where R and R are hydrocarbon groups. Carboxylic acids take their names from their parent alkanes.
For example ethane is the parent alkane of ethanoic acid. General formula of acids is. Carboxylic acids are the most common type of organic acid.
You can the have di-carboxylic acids. The general molecular formula for carboxylic acid is C n H 2n1 COOH. Ethanoic acid has the formula CH 3 COOH and this structure.
In general dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acidsDicarboxylic acids are also used in the preparation of copolymers. Carboxylic acids are weak acids which react in the same way as dilute mineral acids. General formula The general formula for the carboxylic acids is C n H 2n1 COOH where n is the number of carbon atoms in the molecule minus 1.
The general molecular formula for carboxylic acids is C n H 2n1 COOH. Answer 1 of 3. The general formula of a carboxylic acid is R-COOH were COOH refers to the carboxyl group and R refers to the rest of the molecule to which this group is attached.
There are five different compounds with this general formula where the only. A carboxylic acids general formula is R-COOH where COOH denotes the carboxyl group and R denotes the rest of the molecule to which this group is attached. Carboxylic acid any of a class of organic compoundsin which a carbonC atom is bonded to an oxygenO atom by a double bond and to a hydroxyl group OH by a single bond.
Carboxylic acids contain at least one carboxyl group. A carboxylic acid group always lies at the end of a carbon chain. Definition A carbon oxoacid acid carrying at least one COOH group and having the structure RCOOH where R is any any monovalent functional group.
There is carbon in this carboxyl group that has a double connection with an oxygen atom and a single bond with a hydroxyl group. This is what we call trying too hard. K e q R C O O H 3 O H 2 O R C O O H K a K e q H 2 O R C O O H 3 O R C O O H Where K e q is the equilibrium constant and K a is the ionization constant of the acid or dissociation constant of the acid.
The accepted general formula for. For acetic acid it is CH3. For formic acid it is H.
Carboxylic acids are organic molecules which form an homologous series with the general formula C n H 2n O 2. For n1 we get C H 3. In general carboxylic acids are represented by the formula RCOOH where R is a hydrocarbon group.
What is a Carboxylic Acid. The general molecular formula of the carboxylic acid homologous series is rmC_rmnrmH_2rmnrmO_2 where rmn 123 ldots. They are named like hydrocarbons according to the number of carbon atoms in the molecule.
A dicarboxylic acid is an organic compound containing two carboxyl functional groups COOH. A carboxylic acids general formula is R-COOH where the COOH formula denotes the carboxyl group and R denotes the rest of the molecule to which this group is linked. Name of Carboxylic Acid.
Oxalic acid for example is a carboxylic acid where R is also a carboxylic acid. A fourth bond links the carbon atom to a hydrogenH atom or to some other univalent combining group. There is carbon in this carboxyl group that has a double bond with an oxygen atom and a single bond with a hydroxyl group.
The general molecular formula for dicarboxylic acids can be written as HO 2 CRCO 2 H where R can be aliphatic or aromatic. Carboxylic acids are nothing but organic compounds in which the carbon atom is. Carboxylic acids with two or more carboxyl groups attached are called.
These particles are constituents of the nucleus of all elements except for hydrogen H. Definition of neutron - Chemistry Dictionary.

Rutherford Model Definition Facts Atomic Structure Atom Neon Atom
They can be diffracted like photons yet can collide with each other and other particles like other matter.

Definition of neutron in chemistry. Neutrons can exist in a free state. These stars would be supported by the exclusion principle repulsion between neutrons and protons rather than between electrons. A part of an atom that has no electrical.
An atoms mass number. A part of an atom that has no electrical charge 2. Neutrons are the largest of the particles that make up the atom.
All atoms are electrically neutral because every atom has an equal number of electrons and protons. It can have a variable number of neutrons forming isotopes since neutrons do not carry a net electric charge. Neutron in Chemistry topic.
Return to top of page. They were therefore called neutron stars. The scientist James Chadwick is the one who successfully discovered this atomic part.
What is a Neutron. Its mass is slightly bigger than the protons mass but higher than the electrons mass. Neutrons are produced by fission reactions in a nuclear reactor or by irradiating a metal target with high-energy protons from an accelerator which is called spallation.
The definition of a neutron is a nucleon with no charge. He found neutron particle on his experiment in the 1932. Atoms are generally spherical although there are.
It is proposed to amend the definition of a measurand to include exact identification of the entity to be determined and specification of the system to which the measurement is intended to apply. Search the Dictionary for More Terms. It is almost identical in massto a proton but carries no electric charge.
A part of an atom that has no electrical charge Examples from the Corpus neutron First then what about neutron decay. The neutron is a baryon which means it is a massive particle that is made up of 3 quarks 1 up quark and 2 down quarks. A neutral atom has the same number of protons and electrons.
Electrons have properties of both particles and waves. Mass number number of protons number of neutrons. Neutrons are in the nucleus the center of the atom.
Neutron has the mass of 16750 X 10-27 kg. An example of a neutron is something that turns into a proton and electron. Neutrons are part of the nucleus of all atoms except hydrogen and have a mean lifetime of approximately 10103 seconds as free particles.
2 a particle of matter that has a mass of 1009 amu but no electrical charge. Nearly all of the atoms massis located in the nucleus. Reactor sources produce a continuous spectrum of neutron energies and require a monochromator crystal to select a particular energy.
Most of the atoms volume holds the electron cloud whose mass is tiny. 1 A neutral subatomic particle having a mass of 10087 amu. Is the total number of protons and neutrons.
An uncharged elementary particle that has a mass nearly equal to that of the proton and is present in all known atomic nuclei except the hydrogen nucleus. A neutron is the particle in the atomic nucleus with a mass of one and a charge of zero. A particlefound in the nucleusof an atom.
The number of neutrons in an atom determines its isotope. The nucleus is tiny compared with the total size of the atom.
TEMs have a maximum magnification of around 1000000 but images. These fine mesh grids are carefully checked.
The first crude electron microscope was capable of magnifying objects 400 times.

Magnification of transmission electron microscope. Transmission Electron Microscopy magnification. In order to get a better idea of just how small that is think of how small a cell is. AThe TEM uses light and magnification to observe details of objects.
Nasseri and Mohammadi 99 obtained individual cellulose whiskers with length L of 8728 nm and diameter d of 153 nm with an average aspect ratio Ld of whiskers obtained was 62. X 50 to X 1000000 14 Ångstom resolution LaB6 source backscattered and secondary electron detectors Gatan Digi-PEELS Electron Energy Loss Spectrometer software and off axis imaging camera Kevex Quantum 10 mm2 X-ray detector detects elements down to boron. Transmission Electron microscope - Principle Construction Working Advantages and Disadvantages Electrons are made to pass through the specimen and the image is formed on the fluorescent screen either by using the transmitted beam or by using the diffracted beam.
The scanning electron microscope SEM has a large depth of field so can be used to examine the surface structure of specimens. TEMs have a maximum magnification of around. The scanning electron microscope is used to visualise the surfaces of cells and even whole organisms.
A scanning transmission electron microscope has achieved better than 50 pm resolution in annular dark-field imaging mode and magnifications of up to about 10000000 whereas most light microscopes are limited by diffraction to about 200 nm resolution. The maximum magnification of light microscopes is usually 1500 and their maximum resolution is 200nm due to the. The transmission electron microscope TEM is used to examine thin slices or sections of cells or tissues.
Transmission electron microscopy TEM analysis is conducted to get the actual size of the nanocrystalline cellulose fibers and in some cases the morphology. The transmission electron microscope is used to view thin specimens tissue sections molecules etc through which electrons can pass generating a projection image. A calibration sample for transmission electron microscopy TEM has been developed that performs the three major instrument calibrations for a transmission electron microscope.
A Transmission Electron Microscope TEM produces a 2D image of a thin sample and has a maximum resolution of 500000. Jiang and Hsieh obtained cellulose NFs with. Calibration Specimens for Transmission Electron Microscopy STEM Magnification Calibration Fine Mesh Grids.
Electron microscopes are normally built underground in order to reduce interference in the form of vibrations from environmental. The image magnification calibration for measurements of images the camera constant calibration for indexing diffraction patterns and the imagediffraction pattern rotation calibration for relating crystal directions. A Transmission Electron Microscope TEM utilizes energetic electrons to provide morphologic compositional and crystallographic information on samples.
The first practical electron microscope was built by in 1938and had 10 nm resolution. There are different types of Electron Microscope. The electron microscope isnow an integral part of.
Although modern electron microscopes can magnify an object 2 million times they are still based upon Ruskas prototypeand his correlation between wavelength and magnification. The transmission electron microscope TEM gives the highest magnification and resolution and it is used to observe the internal ultrastructure of cells. TEMs can magnify objects up to 2 million times.
Available as a sandwich in a folding 305 mm mesh grid or in a square mesh 25 mm. There are different types of Electron Microscope. Electron Microscopes can have magnifications of 500000.
Transmission electron microscopes TEM are microscopes that use a particle beam of electrons to visualize specimens and generate a highly-magnified image. Whereas the SEM produces images on a computer that can be colorized to enhance detailsbThe SEM is capable of magnification up to 1000000 X whereas the TEM magnifies an object up to 100000 XcThe TEM is useful for analyzing the internal organelles of a cells while the SEM gives a 3-D view of the external. The method is also known as SEM analysis and SEM microscopy and is used very effectively in microanalysis and failure analysis of.
Electron Microscopes can have magnifications of 500000. Unlike glass lenses the resolution and magnification of an electromagnetic lens is affected by changing the current through the lens whereas in a light microscope it is done by mechanically changing the lenses. They are suitable for the low magnification range of a TEM.
Scanning Electron Microscopy SEM is a test process that scans a sample with an electron beam to produce a magnified image for analysis. Where a scanning electron microscope. At a maximum potential magnification of 1 nanometer TEMs are the most powerful microscopes.
A transmission electron microscope TEM is a special type of microscope that uses electrons to create a magnified image up to 1000000x. A Transmission Electron Microscope TEM produces a 2D image of a thin sample and has a maximum resolution of 500000. What is the magnification and resolution of a light microscope.
Fewer electrons per unit area equates to a lower signal and lower signal-to-noise and if one needs or wants to severely limit the electron dose in terms of e-Å 2 there comes a point where the magnification is so high and the dose is so low that there is effectively no signal left at the detector.
I TEM analysis is one of the few methods. Medical Definition of transmission electron microscope.

Sem Vs Tem Electron Microscopy Microbe Online
Transmission electron microscope TEM an electron microscope that produces highly magnified images of ultrathin tissue sections or other specimens.
Definition of transmission electron microscope. Specially prepared materials samples may. They are also the most powerful microscopic tool available to-date capable of producing high-resolution detailed images 1 nanometer in size. This increased resolution allows us to study ultrastucture of organelles viruses and macromolecules.
The Transmission Electron Microscope TEM Similar to the general scheme of a light microscope a transmission electron microscope 1 2 consists of an electron source a condenser system an objective lens and a projector system as shown in Fig. TEMs are costly large cumbersome instruments that require special housing and maintenance. A conventional electron microscope which produces an image of a cross-sectional slice of a specimen all points of which are illuminated by the electron beam at the same time compare scanning electron microscope.
Transmission electron microscopes TEM are microscopes that use a particle beam of electrons to visualize specimens and generate a highly-magnified image. Transmission electron microscope TEM - definition of transmission electron microscope TEM by The Free Dictionary. Transmission electron microscope noun A form of electron microscope in which an image is derived from electrons which have passed through the specimen in particular one in which the whole image is formed at once rather than by scanning.
The transmission electron microscope TEM uses a high voltage electron beam emitted by an electron gun. An optical instrument that uses a lens or a combination of lenses to produce magnified images of small objects especially of objects too small to be. It can be considered as a golden standard for the characterization of NM for several reasons 1.
Since the wavelength of an electron is much smaller than the wavelength of visible light diffraction effects occur at much smaller physical dimensions. Many transmission electron microscopes have additional instruments attached to it such as an. Microscopio electrónico de transmisión instrumento usado para visualizar las células con una capacidad superior de un millón más de visualización que el microscopio común.
1Many transmission electron microscopes have additional instruments attached to it such as an X-ray detector andor an energy loss spectrometer in order to be able to perform elemental. A Transmission Electron Microscope produces images via the interaction of electrons with a sample. Transmission electron microscope TEM type of electron microscope that has three essential systems.
An electron beam passes through the metal-impregnated specimen and is focused by magnetic lenses into an image. The transmission electron microscope TEM operates on many of the same optical principles as the light microscope. In order to get a better idea of just how small that is think of how small a cell is.
1 an electron gun which produces the electron beam and the condenser system which focuses the beam onto the object 2 the image-producing system consisting of the objective lens movable specimen stage and intermediate and projector lenses which focus the electrons passing through. Similar to the general scheme of a light microscope a transmission electron microscope 1 2 consists of an electron source a condenser system an objective lens and a projector system as shown in Fig. Transmission Electron Microscope TEM definition.
Electromagnetic lenses are used to focus the electron beam on the sample. As the electron beam passes through the sample and the atoms. What is a Transmission Electron Microscope.
Transmission electron microscope TEM an electron microscope that produces highly magnified images of ultrathin tissue sections or other specimens. The TEM is analogous in many ways to the conventional compound light microscope. English-Spanish Medical Dictionary Farlex 2012.
An electron beam passes through the metal-impregnated specimen and is focused by magnetic lenses into an image. The TEM has the added advantage of greater resolution. Definition of Transmission Electron Microscopy TEM Electron microscopy is an imaging technique that uses an electron beam to probe a material.
A transmission electron microscope is an electron microscope used to see objects far smaller than cells. Transmission electron microscopy TEM is a versatile technique to analyse the size morphology crystallographic structure and chemical composition of a wide range of nanomaterials NM. In a Transmission electron microscope the electron beam is transmitted through a very thin specimen or object and forms a highly magnified and detailed image of the sample.
TEMs can magnify objects up to 2 million times. This microscope uses electron beams instead of light. The transmission electron microscope TEM The transmission electron microscope is used to view thin specimens through which electrons can pass generating a projection image.
Transmission electron microscope TEM and scanning electron microscope SEM work on the same basic principle. Electron gun is a heated tungsten filament which emitted electron beams.

Visualization Of The Cell Using Em Scanning Electron Microscope Microscopic Photography The Cell
What Is An Electron Microscope Definition Types Uses Study Com.

Parts of transmission electron microscope. The beam of electrons are the focused on the specimen by the. Electron microscope definition. By connecting this gun to a high-voltage source of about 100 300 kV the gun begins to emit electrons by either thermionic or field electron emission.
Instead of detecting electrons being transmitted from an electron source Scanning Electron Microscopy uses the primary electron beam to excite the specimen. Electron Microscopes contain these following parts. Transmission electron microscope TEM type of electron microscope that has three essential systems.
Electron source Electromagnetic lens system Sample holder Imaging system The electron source is an electron gun which consists of a tungsten filament. The TEM consists of an electron emission source which may be a tungsten filament or a lanthanum hexaboride LaB 6 source known as electron gun. DIFFERENCE BETWEEN OPTICAL MICROSCOPE AND ELECTRON MICROSCOPE.
It is the source of electrons. Air needs to be pumped out of the vacuum chamber creating a space where electrons are able to move. The organization of the transmission electron microscope TEM is similar to that of the light microscope.
An electron microscope consists of an electric gun microscope column electromagnetic coils a fluorescent screen and some other accessories described below. Transmission electron microscopes TEM are microscopes that use a particle beam of electrons to visualize specimens and generate a highly-magnified image. Simple Microscope Parts Functions Diagram And Labelling.
Scanning-transmission electron microscopes irradiate the sample in a sequential raster pattern like scanning electron microscopes but still form images from those electrons that are transmitted through the specimen ie the electron detector is on the far side of the specimen unlike the case for scanning electron microscopes. Electron Microscope is scientific instrument that use a beam of highly energetic electrons to examine objects on a very fine scale ODUCTION. This filament emits electrons when it is heated.
It is a special type of microscope having a high resolution of images able to magnify objects in nanometres which are formed by controlled use of electrons in vacuum captured on a phosphorescent screen. It is usually a hairpin-shaped tungsten wire. 1 an electron gun which produces the electron beam and the condenser system which focuses the beam onto the object 2 the image-producing system consisting of the objective lens movable specimen stage and intermediate and projector lenses which focus the electrons passing through the.
Parts of A Transmission Electron Microscope 1. A Transmission Electron Microscope produces a high-resolution black and white image from the interaction that takes place between prepared samples and energetic electrons in the vacuum chamber. The illumination source or electron gun in a thermo-ionic emission TEM works much like a light bulb.
A TEM is composed of the following. Simple Microscope Parts Functions Diagram And Labelling. EM consist of an electron gun as a main source of electron.
The transmission electron microscope TEM was the first type of Electron Microscope to be developed and is patterned exactly on the light transmission microscope except that a focused beam of electrons is used instead of light to see through the specimen. Electron guns consist of four important parts the filament a biasing circuit a Wehnelt cap and an extraction anode. Whereas SEM produces images by detecting secondary electrons which are emitted from the surface of the specimen due to excitation by the primary electron beam.
This technique produces black and white two-dimensional images. Uses optical glass lens 2. It was developed by Max Knoll and Ernst Ruska in Germany in 1931.
Ernst Ruska and Max Knolls discovered the first Transmission Electron Microscope in 1931. Transmission Electron Microscopy Tem. A typical commercial transmission electron microscope TEM costs about 5 for each electron volt eV of energy in the beam and if you add on all available options it can easily cost up to5 for.
Components of Transmission Electron Microscopy. Nasseri and Mohammadi 99 obtained individual cellulose whiskers with length L of 8728 nm and diameter d of 153 nm with an average aspect ratio Ld of whiskers obtained was 62. At first faced with the weak contrast of the early images the operators had to use specific electron-dense contrasting agents to reveal the ultrastructure of their samples.
This is the fundamental principle behind Transmission Electron Microscopy. Abstract Following the first electron micrographs of cotton in 1940 the development of transmission electron microscopy applied to native cellulose has been evolving in a series of successive advances. A The electron gun is located at the top of the body of microscope.
In order to get a better idea of just how small that is think of how small a cell is. There are four parts for a transmission electron microscope. An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination.
Solve This 21 01 File Handbook Of Titration Pdf Free. Transmission electron microscopy TEM analysis is conducted to get the actual size of the nanocrystalline cellulose fibers and in some cases the morphology. Parts of an Electron Microscope.
TEM forms image when radiations pass and are transmitted through the specimen. TEMs can magnify objects up to 2 million times. The transmission electron microscope TEM is used to examine thin slices or sections of cells or tissues the scanning electron microscope SEM has a large depth of field so can be used to.
Jiang and Hsieh obtained cellulose NFs with. A filament cathode is the source of electrons. Nightsea Fluorescence Viewing Systems.
It was thus found.
What is the difference between Saturated and Unsaturated. Linoleic acid olive oil and alpha-linoleic acid nuts like almonds pecans peanuts and cashew canola oil peanut butter peanut oil avocado almond butter 4 7 9 Saturated and unsaturated fats are both needed by the body to remain healthy.
First lets discuss saturated fatty acids.

Saturated vs unsaturated fats chemistry. In saturated hydrocarbons all the bonds are single bonds. Overall saturated compounds are less reactive than unsaturated compounds. When a solution is saturated more solutes cannot be dissolved in it.
One notable exception though is ricinoleic acid found in castor oil which is unsaturated but gives a rich fluffy lather thats quite stable too. Saturation is derived from the Latin word saturare meaning to fill. Difference Between Saturated Fats And Unsaturated Fats.
Saturated fats have the maximum number of hydrogens possible while unsaturated fats have a double bond between two of the carbons. Apparatus and equipment per group w White tile w Conical flask w Dropper pipette. Im wondering how this relates to their chemical structure -- saturated fats contain only single bonds between carbons yet to qualify as an unsaturated fat a CC double bond must exist.
Monounsaturated fats are typically liquid at room temperature and include canola oil and olive oil. Show activity on this post. Saturated fats tend to be solid at room temperature and from animal sources while unsaturated fats are usually liquid and from.
Vegetable oils canola oils and plant oils. From Alila Medical Media. This type of unsaturated fat contains two or more double bonds in their structure.
Unsaturated versus saturated versus Trans fats. Saturated fats would clog arteries that might increase the risk of cardiac disease while unsaturated fats help to keep the proper body functioning. Unsaturated fats come from plants and occur in the following kinds of foods.
Are fatty acids comprised of non-polar hydrocarbon chains that contain double bonds. In chemistry a saturated compound is a chemical compound that resists the addition reactions such as hydrogenation oxidative addition and binding of a Lewis base. Saturated fats lack double bonds between the individual carbon atoms while in unsaturated fats there is at least one double bond in the fatty acid chain.
This gives it a straight structure which allows them to stack on top of each other. Saturated fats contain only singles bonds so their structures are straight Carbon chains. Saturated fatty acids form saturated fats while unsaturated fatty acids form unsaturated fats.
Saturated fatty acids lack double bonds between the individual carbon atoms while in unsaturated fatty acids there is at least one double bond in the fatty acid chain. Poly-unsaturated fats have multiple double bonds are even more bent and have even lower melting points. The difference between saturated and unsaturated fat lies in the number of double bonds in the fatty acid chain.
In saturated fats each carbon has four hydrogens bonded to it. Description The students titrate different oils and fats mixed with Volatile against bromine water. What is the difference between Saturated and Unsaturated Fatty Acids.
In unsaturated hydrocarbons double bonds and triple bonds are also present. Please try again later. They tend to build up along the sides of blood vessels along with other materials and over time they may clog them which may cause a heart attack or a stroke depending on which blood vessel was clogged.
Unsaturated fats which are liquid at room temperature are different from saturated fats because they contain one or more double bonds and fewer hydrogen atoms on their carbon chains. Generally saturated fatty acids will give you a creamy stable lather while unsaturated fatty acids will result in a fluffy but unstable lather. An error occurred while retrieving sharing information.
The chain length of most common fatty acids is of 16-18 number of carbon. Saturated fats are solid under room temperature and unsaturated fats are liquids under room temperature is due the fact that saturated fats has greater intermolecular force and thus higher melting point. Saturated means fully filled whereas unsaturated means no fully filled.
Saturated fats can impact your health. The term is used in many contexts and for many classes of chemical compounds. License this video.
They tend to be liquid at room temperature. Are fatty acids comprised of long non-polar hydrocarbon chains saturated with hydrogen and has no double bonds. Unsaturated fatty acids contain some carbon atoms that bond with each other using double bonds.
Saturated fatty acids have no double CC double bonds in the fatty acid chain and thus the saturated fatty acids have the maximum number of hydrogen atoms. Saturated fatty acids contain carbon atoms that connect with each other in a chain of single bonds. Unsaturated fats are loosely packed.
Saturated and unsaturated fats vary greatly in. There are two main types of unsaturated fat. Saturated fatty acids do not contain double bonds C-C only single bonds whereas unsaturated fatty acids contain one or more double bonds CC.
Im sure most of us have heard that saturated fats are solid at room temperature and unsaturated fats are liquid at room temperature. However unsaturated fats are more digestible and have higher absorption rate and therefore the metabolizable energy that unsaturated fats provide is higher than that provided by saturated fats. They are liquid at room temperature.
The difference between saturated and unsaturated fat lies in the number of double bonds in the fatty acid chain. Classic chemistry experiments Inaugurations in fats and oils Topic Organic chemistry saturated and unsaturated fats. Yes it is a fact that saturated fats contain more gross energy due to higher H concentration.
The triglyceride is considered as the common and simple type of fat having three fatty acids and glyceride. So each carbon atom can bond with two hydrogen atoms and is said to be saturated with hydrogen. Every carbon wants to have four bonds.


