Every compound has its own CHEMICAL FORMULA and its own NAME. 14 disilicon hexabromide Si2Br6.

Answer Predict The Formula For Magnesium Clutch Prep
5 Ag3P silver phosphide.

Magnesium phosphide formula. Magnesium nitride Mg3N2 6. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. Chemical formulas for ionic compounds are called ionic formulas The chemical formula for an ionic compound.
Li 4C lithium carbide Ba 3N 2 barium nitride MgBr 2. For example Cu. The total positive charge must balance the total negative charge.
You should complete this by Sunday. Zinc iodide ZnI2 3. Al 3 Aluminum.
I Magnesium chloride HNO 3 A Nitric acid Ca 3 P 2 I Calcium phosphide ZnCO 3 I Zinc carbonate NaNO 3 I Sodium nitrate H 2 SO 3 A Sulfurous acid AgCl I Silver chloride CH 4 M Carbon tetrahydride Cu 2 C 2 O 4 I Copper I oxalate SnI 4 I Tin IV iodide PbS 2 I Lead IV sulfide CO 2 M Carbon dioxide Al 2 SO 4 3 I Aluminum sulfate. 24304-00-5 AlNO 2 3. P silver phosphide 6 IO 2 iodine dioxide 7 VO 2 vanadium IV oxide 8 PbS lead II sulfide 9 CH 4 methane 10 N 2 O 3 dinitrogen trioxide Write the formulas of the following chemical compounds.
Rubidium and bromine. 3 aluminum oxide Al and O 6 magnesium phosphide Mg and P Formula. The key to writing proper ionic formulas is simple.
Magnesium phosphide Mg 3 P 2 also is moisture sensitive. 2 calcium chloride Ca and Cl 5 sodium nitride Na and N Formula. Ag Silver.
Gallium and oxygen. 5 mercury I phosphide Hg 3P cobalt III phosphide CoP cesium nitride Cs 3N magnesium oxide MgO phosphorus III chloride PC ℓ 3 2. If H is the positive ion the compound is an acid as we will see later on page 6 The positive ion cation may be a monatomic metal ion such as.
Because the charges on the ions are. Copy this to my account. Sodium thiosulfate ____Na2S2O3_____ 3.
11 tetraphosphorus triselenide P4Se3. Use Lewis dot structures to show the covalent bonding in the following pairs of. Chemical formulas for ionic compounds are called ionic formulas.
Aluminum and iodine d. Sodium chloride NaCl and magnesium oxide MgO. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A.
6 IO2 iodine dioxide. 616 Write the symbols for the ions and the correct formula for the a. Again you may be familiar with some of these ions.
Give the formula for the following. A proper ionic formula has a cation and an anion in it. 8 PbS lead II sulfide.
For those with electropositive metals the. The key to writing proper ionic formulas is simple. Name the following binary compounds.
A monatomic meaning one-atom cation takes its name from the name of the element. Lead II chloride PbCl2 5. 12 potassium acetate KC2H3O2.
Sodium and nitrogen c. Write the symbols for the ions and the correct formula for the ionic compound formed by each of the following. CHARGE FORMULA NAME CHARGE FORMULA NAME 1 H Hydrogen 1- H- Hydride Li Lithium F-Fluoride Na Sodium Cl- Chloride K Potassium Br- Bromide Cs Cesium I- Iodide Ag Silver 2 Mg2 Magnesium 2- O2- Oxide Ca2 Calcium S2-Sulfide Sr2 Strontium Se2-Selenide Ba2 Barium Zn2 Zinc Cd2 Cadmium 3 Al3 Aluminum 3- N3- Nitride P3- Phosphide Table 2 Metals That Form More.
Sulfur dioxide ____SO2_____ 2. Na Mg 2 Non. Binary Ionic Compounds Type II The cation of a transition metal is always named first like any cation and the anion second.
An ionic compound is never formed between two cations only or two anions only. Magnesium Mg 2 mercury I Hg 2 2 nickel Ni 2 potassium K rubidium Rb silver Ag sodium Na strontium Sr 2 tin II Sn 2 zinc Zn 2 Examples of Negative Ions. Iron III oxide Fe2O3 4.
An ionic compound is never formed between two cations only or two anions only. Hg23P2 mercury I phosphide Section D Write the formula for the ionic compounds containing transition metals BE CAREFUL TRANSITION METALS MAY HAVE ROMAN NUMERALS and NICKNAMES 1. These species are insoluble in all solvents - they are 3-dimensional solid state polymers.
Superscripts are used to. Use the Stock system where necessary. The transfer of electrons between metals and non-metals produces charged particles called ions.
2 provides a dispersion formula based on data from ref. Just as atoms can lose electrons to become cations some can gain electrons and become negatively charged anions. Complete these in lab and on your own time for practice.
10 N2O3 dinitrogen trioxide. Copper phosphide Cu 3 P illustrates a rare stoichiometry for a phosphide. The nomenclature naming systems for IONIC and MOLECULAR compounds are different.
7 VO2 vanadium IV oxide. Calcium and chlorine b. Potassium and sulfur b.
Use the stock form for the transition metals. Fluoride is sometimes added to community water supplies. Ca 2 Calcium.
Optical constants of CaCO 3 Calcium carbonate Calcite Ghosh 1999. Predicting the Formula of an Ionic Compound In a chemical formula subcripts are used to specify the numbers of a type of atom in the formula. A proper ionic formula has a cation and an anion in it.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. E-mail to a friend. Binary Ionic Formula Practice Name_____ 1 sodium chloride Na1 Cl-1 NaCl 2 lithium bromide Li1 Br-1 LiBr 3 magnesium flouride Mg2 F-1 MgF2 4 potassium oxide K1 O-2 K2O 5 calcium sulfide Ca2 S-2 CaS 6 aluminum iodide Al3 I-1 AlI3 7 barium bromide Ba2 Br-1 BaBr2 8 aluminum sulfide Al3 S-2 Al2S 3 9 calcium phosphide P-3 Ca2 P-3.
11 tetraphosphorus triselenide P 4 Se 3 12 potassium acetate KC 2 H 3 O 2 13 iron II phosphide Fe 3 P 2 14 disilicon hexabromide Si 2 Br 6. 1 barium oxide Ba and O 4 sodium oxide Na and O Formula. Indium phosphide InP and gallium phosphide GaP are used as a semi-conductors often in combination of related arsenides.
Formula 21 sodium phosphide Na 3 P 22 magnesium nitrate MgNO 3 2 23 lead II sulfite PbSO 3 24 calcium phosphate Ca 3 PO 4 3 25 ammonium sulfate NH 4 2 SO 4 26 silver cyanide AgCN 27 aluminum sulfide Al 2 S 3 28 beryllium chloride BeCl 2 29 copper I arsenide Cu 3. 13 iron II phosphide Fe3P2. Data Expressions for n CSV - comma separated TXT - tab separated Full database record Optical transmission calculator.
Metals lose electrons to produce positve ions called cations. 4 copper I phosphide 5 tin IV selenide 6 gallium arsenide 7 lead IV sulfate 8 beryllium bicarbonate 9 manganese III sulfite 10 aluminum cyanide 11 CrPO 4 2 12 VCO 3 2 13 SnNO 2 2 14 Co 2 O 3 15 TiC 2 H 3 O 2 2 16 V 2 S 5 17 CrOH 3 18 LiI 19 Pb 3 N 2 20 AgBr 21 NaBr sodium bromide 22 ScOH 3 scandium III. Iron II fluoride FeF2.
Chemical Formula Nomenclature Practice. These consist of any positive ion except H combined with any negative ion. The total positive charge must balance the total negative.
An ionic compound is composed of a metal and a non-metal. Formula Type Chemical Name HCl A Hydrochloric Acid Hg 2 SO 4 I. Cadmium sulfide CdS 2.
Write the correct chemical formula for the ionic compound that forms. For example O 2 is interpreted as a molecule formed by two oxygen atoms and CH 3 OH is interpreted as a molecule with one carbon four hydrogens and one oxygen. Ionic Compound Naming and Formula Writing List 1.
Write the formulas of the following chemical compounds.

This Is How The Ionic Bond Forms In Magnesium Phosphide Mg3p2 Youtube

How To Write The Formula For Magnesium Phosphide Youtube
The Chemical Thesaurus Reaction Chemistry Database

How To Write The Formula For Magnesium Phosphide Youtube
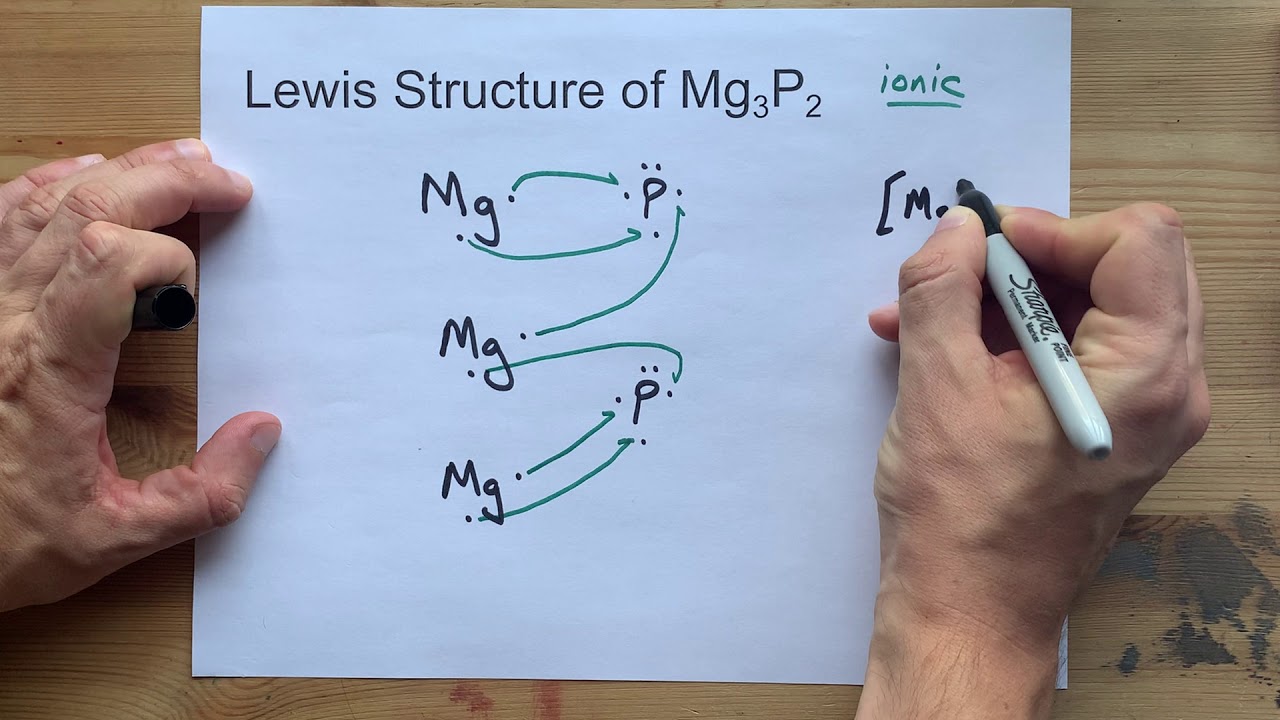
Draw The Lewis Structure Of Mg3p2 Magnesium Phosphide Youtube

This Is How The Ionic Bond Forms In Magnesium Phosphide Mg3p2 Youtube
Magnesium Phosphide Mg3p2 Pubchem