A compound is a substance made up of a definite proportion of two or more elements. This helps scientists to communicate efficiently.

Naming Ionic Compounds Chemistry Worksheets Naming Compounds Worksheet Nomenclature Chemistry
The subscript numbers in an empirical formula should have no common divisor.

Common chemical names and formulas. The following worked examples will look at names and formulae in detail. It is easy to describe elements and mixtures. C 2 H 2.
Common bases are aqueous solutions containing excess hydroxide ions OH. Examples CO2 NaCl. 1 carbon 4 hydrogen.
Chemical formula for water is H₂O which means that water is a compound which is formed by the combination of 2 proportions of H and 1 proportion of O. With the help of Chemical formulas you can check. CaCl OCl blue vitrol.
Potassium aluminum sulfate dodecahydrate. H 2 SO 4. Chemical formulas provide a lot of information about chemical substances such as how many and what atoms they are made of as well as the way the atoms are arranged.
C 6 H 5 NH 2. 1 nitrogen 3 hydrogen. Common Names Formulas and Uses of Important Chemical Compounds.
Common Name Chemical Name Formula. Names of common covalent compounds. 2-propanol propyl alcohol isopropyl alcohol.
H 3 PO 4. Since table salt is an ionic compound the formula implies that numbers of Na ions and Cl- ions are the same in the solid. KAlSO 4 212H 2 O.
List of Chemical Formulas and their Common Names Definition of Chemical Formula Chemical formulas are expressions which state the number and types of atoms present in a molecule of a substance. Ammonium sulfate note the 2 word name 1. Sodium hydrogen carbonate or Sodium bicarbonate.
Writing chemical formulae 1. 100 rows For instance the chemical name of NaCl is Sodium chloride and its common. Ternary acids commonly contain hydrogen a nonmetal and oxygen.
The chemical formula also stands for one molecule of. Similarly sulfuric acid has a chemical formula H₂SO₄ which means that in this compound the relative proportion of H S and O are. Common Chemical Formula List NaCl Sodium chloride H 2 O Water C 6 H 12 O 6 Glucose C 2 H 6 O Alcohol CaSO 4 Sulfate Group H 2 S Hydrogen Sulfide NaCl Salt O 2 Oxygen C 2 H.
263 rows One atom is present in each of the elements hydrogen carbon and nitrogen respectively. The name of the most common form of the acid consists of the nonmetal root name with the -ic ending. Al 2 O 3.
Balance charges if necessary using subscripts. The chemical formula of a covalent molecular substance gives the number of atoms per molecule. CuSO 4 5H 2 O.
The list of all the important Names and Formulas of Common Chemical Compounds is given in the following pages of this Free E-book on Names and Formulas of Common Chemical Compounds. Writing Ionic Compound Formulas Example. Write the formulas for the cation and anion including CHARGES.
The definition of an acid and a base is expanded later in a first-year chemistry course. Na 3 PO 4. A chemical formula clearly shows the composition of a substance.
Bleach laundry Sodium hypochlorite. The acid containing one less oxygen atom than the most common form is designated by the. C 2 H 5 OH.
Again the subscript 1 is omitted. Acetone CH 3 2 CO. Na 2 B 4 O 710 H 2 O.
28 rows Common Names Chemical Compounds Chemical Formula. It not only reveals the identity of the constituent elements but also their fixed ratio by mass. Chemical Formulas NAMES AND FORMULAS OF COMMON ACIDS AND BASES.
Check to see if charges are balanced. Use parentheses if you need more than one of a polyatomic ion. CH 3 COO 5.
The name of a base is the name of the cation followed by the name of the anion hydroxide. Therefore to help you get these important marks we have created a Free E-book on Names and Formulas of Common Chemical Compounds. Compounds have a common name a proper name and chemical formulae.
Formula from names of compounds. For now common acids are aqueous solutions containing excess hydrogen ions H. After reading Lesson 94 answer the following questions.
Chemical substances have names just like people have names. Ethanol or ethyl alcohol. Name of compound Formula Atoms in each molecule.
Al 2 O 3 2H 2 O. The formula of a base is written by showing the number of hydroxide ions needed to balance the positive charge on the cation. Formulas of Ternary Acids.
The formula of a covalent network or ionic compound gives the. 1 carbon 2 oxygen. Sulfuric acid 98072 gmol.
CuSO 45 H 2 O. NH 4 SO 4 2- 2. Common used name trade names Chemical Name Chemical Formula.
HNO 3 3 HCl.
A hygroscopic organic acid C 3 H 6 O 3 present normally especially in muscle tissue as a by-product of anaerobic glycolysis produced in carbohydrate matter usually by bacterial fermentation and used especially in food and medicine and in industry. When milk sugar lactose undergoes fermentation the product obtained is lactic acid.

Pin By Kim On Inspiration Power Of Positivity Preventative Health Breathwork
It is also found in sour milk.

Definition of lactic acid. The definition of lactic acid is an organic acid C 3 H 6 O 3 present especially in muscle tissue as a by-product of anaerobic glycolysis produced in carbohydrate matter usually by bacterial fermentation and used especially in food and medicine and in industry. Some tissues can use lactate as a substrate and oxidize it to carbon dioxide C02 and water but only the liver and kidney have the necessary enzymes to utilize lactate for the process of gluconeogenesis. Lactic acid is also produced by muscle tissue during exercise especially when oxygen supply is limited and can cause cramping pains.
Lactic acid is produced when glucose is metabolized and an excessive amount may irritate muscles. Lactic acid or lactate is a chemical byproduct of anaerobic respiration the process by which cells produce energy without oxygen around. It is then metabolized mostly via the liver and the kidney.
The acidity inhibits the GROWTH of competing bacteria. These bacteria are used in the food industry for example in the preparation of dairy products pickles and sauerkraut but they can also cause food spoilage. A byproduct of carbohydrate metabolism anaerobic metabolism.
What is lactic acid and why is it important. Medical Definition of Lactic acid. Definition of lactic acid.
A lactic acid level may be measured in the bloodstream in conditions of metabolic acidosis. Running fast can lead to a build-up of lactic acid in your muscles causing cramp. It is used as a flavoring and preservative for foods.
Lactic Acid DL- is the racemic isomer of lactic acid the biologically active isoform in humans. Lactic acid is also in our. Lactic acid is the waste product produced during anaerobic respiration.
Physiology Lactic acid is an acid which is produced in muscles when glucose is metabolized. A yellowish or clear syrupy organic acid CH 3 CHOHCOOH produced by the fermentation of lactose when milk sours or from sucrose and some other carbohydrates by the action of certain microorganisms and used in tanning leather as. Lactic acid or lactate is a chemical byproduct of anaerobic respiration the process by which cells produce energy without oxygen around.
In lactic acidosis the liver is unable to remove excess acid. What does Lactic acid mean. It is found in cottage cheese leban sour milk yogurt and Koumiss.
Lăktĭk An organic acid produced when milk sours or various fruits ferment. Normally the level of lactic acid in the blood is low. Lactic acid definition a colorless or yellowish syrupy water-soluble liquid C3H6O3 produced during muscle contraction as a product of anaerobic glucose metabolism abundant in sour milk prepared usually by fermentation of cornstarch molasses potatoes etc or synthesized.
Bacteria produce it in yogurt and our guts. During exercise energy expenditure is faster than the oxygen supplied to the muscle cells. Definition of lactic acid bacterium.
Lactic Acid is an organic acid with chemical formula C 3 H 6 O 3. It is also known as milk acid. This reaction in addition to producing lactic acid also produces nicotinamide adenine dinucleotide NAD that is then used in glycolysis to produce energy source adenosine triphosphate ATP.
This test measures the level of lactic acid also known as lactate in your blood. What is Lactic Acid. Lactic acid or lactate is produced during fermentation from pyruvate by lactate dehydrogenase.
Lactic acid is a substance made by muscle tissue and by red blood cells which carry oxygen from your lungs to other parts of your body. Lactic acid in American English. Lactic acid is an intermediate product of carbohydrate metabolism and is.
Lactic acid also causes tooth decay. Lactic acid is normally produced in excess by about 20 mmolkgday which enters the bloodstream. In this starch or sugar is converted into lactic acid by yeast strains and bacteria.
Used chiefly in dyeing and textile printing as a flavoring agent in food and in medicine. This results in the formation of lactic acid and painful muscles. Meaning of Lactic acid.
Lactic acidosis is a form of metabolic acidosis that begins in the kidneys. Any of various bacteria chiefly of the genera Lactobacillus and Streptococcus that produce predominantly lactic fermentation on suitable media and some of which are used in the commercial production of lactic acid and as cheese and butter starters. Lactic acid bacteria a group of Gram-positive BACTERIA see GRAMS STAIN that carry out a LACTIC ACID FERMENTATION in which SUGARS such as LACTOSE are converted to lactic acid.
An organic acid CH3CHOHCOOH produced from pyruvic acid as a result of ANAEROBIC RESPIRATIONin microorganisms see LACTOSEand in the active muscles of animals see OXYGEN DEBT the hydrogenation of pyruvate being catalysed by lactic dehydrogenase LDH. Information and translations of Lactic acid in the most comprehensive dictionary definitions resource on the web.
Available in technical and Food grades. The compound is frequently derived from sugar cane sugar beets and corn.

Know The Chemical Formula Of Sugar Chemical Formula Chemical Structure Molecular
Pure fructose has a sweet taste similar to cane sugar but with a fruity aroma.

Chemical formula of fructose. Fructose is a very sweet white odorless crystalline solid. Bakery confectionery and medicines. It is also commercially manufactured.
Chemistry 21062019 22. A gravity only b inertia c convection and gravity d the sun theres no option for science so i picked chemistry. It also occurs in certain vegetables.
The molecule formula of Chemical. What is the chemical formula fructose. The chemical formula of high fructose corn syrup is roughly 45 C 6 H 12 O 6 55 C 6 H 12 O 6 a q It is unfortunate that both glucose and fructose have the same chemical formula but its just a mix of the two.
What is driving behind plate tectonics plate movment. Fructose a member of a group of carbohydrates known as simple sugars or monosaccharides. What is the molecular formula of fructose.
The mix can vary but 55 fructose 45 glucose is. So in 100g fructose there are. Molecular and Structure Formula Of Fructose.
It is flammable and non-poisonous. Its density is 1694 gcm3. Fructose is present in the form of white crystals.
Fructose is a hexose however it exists as a 5-member hemiketal ring. 103 to 105 C. It is odorless but tastes very sweet.
D-Fructose C6H12O6 CID 2723872 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. Fructose is a covalent chemical compound of carbon hydrogen and oxygen atoms. Fructose is a sugar found naturally in fruits and honey.
Glucose and fructose have the same molecular formula C6H12O6 but glucose has a six member ring and fructose has a five member ring structure. C 6 H 12 O 6 Physical and Chemical Properties of Fructose The carbohydrate can be fermented anaerobically with the help of yeast or bacteria in which they are converted into carbon dioxide and ethanol. Fructose or fruit sugar is a monosaccharide like glucose.
CH_2O The empirical formula is the simplest whole number ratio defining constituent atoms in a species. D---Fructose C6H12O6 CID 5984 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. It is used in.
Fructose is a levorotatory monosaccharide which means it rotates the plane-polarized light in the left direction. Although fructose is a hexose 6 carbon sugar it generally exists as a 5-member hemiketal ring a furanose. Fructose or levulose is a levorotatory monosaccharide and an isomer of glucose C6H12O6.
The chemical composition of fructose is C6H12O6. Substances such as these three which have identical molecular formulas but different structural formulas are. 1 Show answers Another question on Chemistry.
Without purification itll also have dimers trimmers and longer short chains of glucosein small quantities. Prepared by the starch from fruits and corn. C6H12O6 C 6 H 12 O.
4000g1201gmol-1 333molC 672g100794gmol-1 666molH 5329g15999gmol-1 333molO If we divide. Fructose formula is C 6 H 12 O 6. Its structure is represented by formula I while its actual composition is formula II.
C6H12O6 FructoseFormula The chemical composition of fructose is C6H12O6. Fructose is a 6-carbon polyhydroxyketone. Fructose along with glucose occurs in fruits honey and syrups.
The chemical composition of fructose is C 6 H 12 O 6 but shows different bonding from glucose. The chemical formula of Fructose is C6H12O6. Fructose has the same chemical formula as glucose the most common sugar in foods but a different arrangement of the atoms gives it somewhat different properties.
Its naturally found in fruit honey agave and most root vegetables. Three common sugarsglucose galactose and fructose share the same molecular formula. Because of their six carbon atoms each is a hexose.
It is a component along with glucose of the disaccharide sucrose or common table sugar. Its molar mass is 18016 g-mol-1. Its structure is represented by formula I while its actual composition is formula II.
Fructose Formula is C 6 H 12 O 6. Glucose and fructose are monosaccharides and sucrose is a disaccharide of the two combined with a bond. It is a simple type of sugar molecule carbohydrate.
The known molecular formula of fructose is. It will have almost no fructose. Structure formula of fructose.
In all of these problems it is useful to assume 100 g of compund and calculate the ATOMIC composition. Fructose is known as the fruit sugar as its make source in the diet is fruits and vegetables. Moreover its commonly added to processed foods in the.
Pure fructose has a sweet taste similar to cane sugar but with a fruity aroma.
Alcohols work as suitable solvent for many organic compounds. If the carbon that is bonded to the -OH is bonded to three carbons the alcohol is a tertiary 3o alcohol.

The Chemistry Of Alcohol Imgur Chemistry Classroom Chemistry Science Chemistry
Alcohols are a chemical family that includes compounds with one or more hydroxyl -OH groups bound to a single bonded alkane.

Sources of alcohol organic chemistry. See how to rank them using the CARIO method and practice examples every step of the way. Reactions Mechanisms Multiple Bonds. Carbon and two hydrogens the alcohol is a primary 1o alcohol.
Alcohols are amphiprotic making them both acids and bases. Resonance Acidity of Phenol. Struct synth landscapedocx Page 1 Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water.
The addition of RMgX on carbonyl compounds along with hydrolysis gives us alcohols. Identical to a pure sample of Vitamin C isolated from a natural source such as an orange or. Complete your research with top quality products.
Once its saturated decant the liquid into the separatory funnel Extracted with 5 mL of methylene chloride and saved the organic layer then extracted the aqueous layer one more time with an additional 5 mL of methylene chlorideombine the organic layers and dry over anhydrous sodium sulfate. Hydroboration Hydrolysis of alkyl halides Chapter 8 nucleophilic substitution Reaction of Grignard or organolithium reagents with ketones aldehydes and esters. Ethanol is produced industrially by fermentation of glucose.
If the carbon that is bonded to the -OH is bonded to two carbons and one hydrogen the alcohol is a secondary 2o alcohol. Science Organic chemistry Alcohols ethers epoxides sulfides Alcohol nomenclature and properties. We can obtain the three types of monohydric alcohols primary secondary and tertiary alcohols by using Grignard reagents and carbonyl compounds.
More than 50 organic compounds have been isolated from the oil that gives rise to the characteristic odor of a rose. With any of these reactions simply assume an abundance because stoichiometry is not needed in organic chemistry. Figure 913 Sources and uses of common carboxylic acids.
A Review and a Preview. Primary alcohols can be oxidised to aldehydes which can be further oxidised to carboxylic acids. Alcohols are useful in organic chemistry because they can be converted into and out of a variety of other compounds.
Shunji WatariEncyclopædia Britannica Inc. Alcohols are acidic in nature. They react with metals such as sodium potassium etc.
These experiments show that alcohols react similarly in all these reactions. Ad Enabling you to solve the toughest problems in life science. Primary alcohols are more acidic than secondary and tertiary alcohols.
Acidified potassium dichromateVI is a suitable oxidising agent. Alcohols are an important class of molecules with. 156 Reactions of Alcohols.
3 Preparation of Alcohols from Grignard Reagent. The Grignard reagent is basically an organometallic compound. 157 Conversion of Alcohols to Ethers Primary alcohols are converted to ethers on heating in the presence of an acid catalyst usually sulfuric acid.
This video reveals WHY behind physical property trends of alcohol such as solubility boiling point polarity and more. Leaving groupIn organic chemistry the species that leaves the parent molecule following a substitution reaction. Alcohols Diols and Thiols 151.
One of the most abundant of these compounds is known by the common name citronellolUse the systematic nomenclature to name this alcohol. Because -OH is the functional group of all alcohols we often represent alcohols by the general formula ROH where R is an alkyl group. Complete your research with top quality products.
Alcohols with the hydroxyl bound directly to an aromatic benzene ring are called phenols. Alcohols are usually classified as primary secondary and tertiary. They make clear the concept of functional group in organic chemistry.
It links alcohols with aldehydes ketones and carboxylic acids shown below. Organic chemistry describes the structures properties preparation. Simple distillation can be used when the liquids to be separated have boiling points that are quite different.
Ad Enabling you to solve the toughest problems in life science. The conditions for this process. Sources of Alcohols please read Hydration of alkenes Chapter 6 1.
It is due to the polarity of bond between hydrogen atom and oxygen atom of hydroxyl group. Organic Chemistry with a. For example an alcohol is an organic compound with a hydroxyl -OH functional group on an aliphatic carbon atom.
Both alcohols are oxidised to aldehydes which have a sour but fruity smell. C 2 H 5 OH O CH 3 CHO ethanal H 2 O C 3 H 7 OH O CH 3 CH 2 CHO propanal H 2 O. The oxidation of different alcohols is an important reaction in organic chemistry.
Haloalkanes Alcohols Ethers and Amines 4. ORGANIC LABORATORY TECHNIQUES 10 101 DISTILLATION NEVER distill the distillation flask to dryness as there is a risk of explosion and fire. Lets start with physical properties of alcohols and so were going to compare in this case alcohols to alkanes in this alkane on the left here two carbons so this is of course ethane on the right if we take off one of those hydrogens and replace it with an O H right we of course have ethanol right here so lets lets start with boiling point so the boiling point of ethane is approximately negative 89 degrees Celsius and.
As organic compounds are insoluble in water. Acid catalyzed hydration 2. The most common methods of distillation are simple distillation and fractional distillation.
Peppermint Mentha piperita is a natural source of menthol. All of these alcohols. The general formula -OH is used to describe alcohols.
Alcohols are organic compounds in which the hydroxyl functional group -OH is bound to a carbon atom. Cholesterol found in most animal tissues and in egg yolks contains a hydroxyl group OH making it a naturally occurring source of alcohol.
A r or atomic weight is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass. Relative atomic mass Ar.

3 Ways To Calculate Atomic Mass Wikihow
Relative atomic mass or atomic weight is the average atomic mass divided by one unified atomic unit.
Relative atomic mass definition chemistry. Chemistry the ratio of the average mass per atom of the naturally occurring form of an element to one-twelfth the mass of an atom of carbon-12. It is the ratio of the average mass per atom of an element from a given sample to 121. In other words the standard unit is defined by the carbon 12 atom which is assigned a value of 12 units.
The mass number of any one atom will be a whole number. The relative atomic mass of C is 120000 amu whereas that of H is 1008. It is denoted as A r.
So average atomic weight of carbon is 12011 12 u 1 u 12011 12. The Relative Atomic Mass RAM. Relative Atomic Mass Ar The size of atoms is so tiny that we cannot really compare their masses in conventional units such as kilograms or grams so a unit called the relative atomic mass Ar is used.
Relative atomic mass Average mass of the isotopes of the element112th of the mass of one Carbon- 12 atom. In this way the relative atomic mass of any element can. It can be found in any chemistry school textbook.
Isotopes and relative atomic mass. The relative atomic mass Ar of an element is the average mass of one atom of the element when compared with 112 of the mass of an atom of carbon-12 which taken as 12 units. In other words a relative atomic mass tells you the number of times an average atom of an element from a given sample is heavier than one-twelfth of an atom of carbon-12.
Similarly relative atomic mass can be defined in two exactly equivalent ways. The relative atomic mass of an element is the ratio between the average mass of its isotopes to 112th part of the mass of a carbon 12 atoms. The relative atomic mass is the average mass number.
The relative atomic mass of an element is the weighted average of the masses of its isotopes on a scale on which a carbon-12 atom has a mass of exactly 12 units. The mass of a carbon- 12 atom. A r is a measure of how heavy atoms are.
Relative atomic mass symbol. First definition is A_mathrm r is the mass of 1 atom of an element relative to 112 the mass of carbon-12 atom. Relative atomic mass A r.
The relative atomic mass A r of an element is the ratio of the average mass of the atoms of an element to the unified atomic mass unit. Be calculated given the percentage of each isotope. Second definition is what I just found and dont understand.
For all of the isotopes of that element. The relative atomic mass of an element is a weighted average of the masses of the atoms of the isotopes because if there is much more of one isotope then that will influence the average mass. It states A_mathrm r is the mass of 1 mole of atoms relative to 1 mole of carbon-12 atoms.
Relative atomic mass Ar. The relative atomic mass is determined by using the average mass of the isotopes of a particular element. The masses of the atoms vary from 10 -24 to 10 -23 grams.
Everthing else is measured with respect to this atom and is then described as relative atomicmolecular mass. Mass of one atom of 12C average mass of naturally occurring atoms of an element. The relative atomic mass of an element is a weighted mean mass of the isotopes of an element compared with that of the 12 C isotope which has a mass of.
I found out there are two definitions of relative atomic mass A_mathrm r. The relative atomic mass unit is equal to 112th the mass of a carbon-12 atom. It is the 112th of the mass of one atom of 12 C.
The relative mass of an atom is its mass compared to the 12 C isotope 12 atomic mass units. Hence option A is correct. The mass of an atom when compared to another is known as the relative atomic mass Ar.
The A r has no units as it is a ratio and the units cancel each other out. Im using the version of the definition which I find easier Relative atomic mass is given the symbol A r. All other elements are measured by comparison to the mass of a.
A relative atomic mass also called atomic weight. The relative atomic mass is the weighted average of the masses of the isotopes on a scale on which the mass of a carbon-12 atom is exactly 12 units. It is the ratio of the average mass per atom of an element from a given sample to 112 the mass of a carbon-12 atom.
The average atomic weight is dimensionless quantity while atomic mass has the dimension of unified mass unit u But both has the same numerical value. Instead to describe the mass of a single atom scientists use the relative atomic mass which is a ratioLike how we compared milk tea to bread slices we compare an atom to a 112th slice of a carbon-12 atomFor example a hydrogen atom has 1 slices worth of mass a carbon atom 12 slices worth and a magnesium atom 24 slices worth. Weighted average is also called weighted mean.
The weighted mean mass of an atom of an element compared to the mass of 112th a carbon-12 atom. The relative atomic mass is expressed in atomic mass unit amu. Relative atomic mass A r.
The sum of the atomic masses of all the atoms of each of the elements within a molecule. Relative atomic mass is the mass of an atom of an element as a multiple of the standard atomic mass unit.
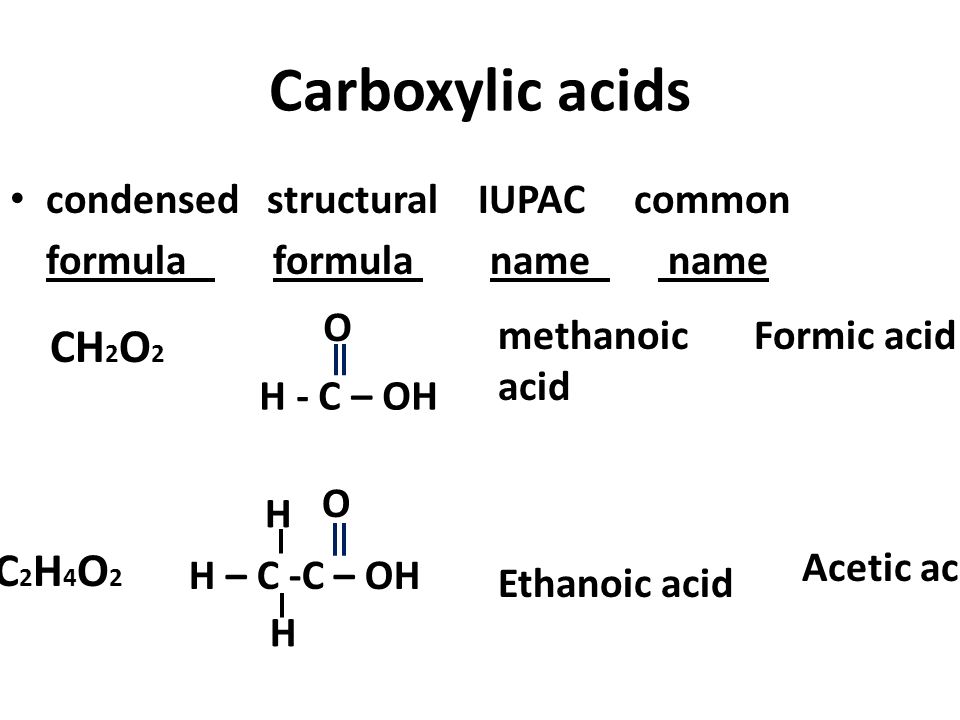
Acetic acid is a simple carboxylic acid consisting of the methyl group CH 3 linked to the carboxylic acid group COOH. Acetic acid also known as ethanoic acid and methanecarboxylic acid is a colorless liquid that has a strong and distinct pungent and sour smell.
Structural Formula Of Carboxylic Acid
Look here to see the condesed structural formula of stearic acid.

Acetic acid condensed structural formula. The condensed structural formula of the compound acetic acid is CH3COOH. It is also called glacial acetic acid ethanoic acid or methane carboxylic acid. Condensed structural formula of t-butyl alcohol.
What Is The Condensed Structural Formula For octanol and ethanoic acid. Its chemical formula is C2H4O2 it has two carbon C atoms four hydrogen H atoms and two oxygen O atoms. We cant draw condensed structural formulas for the rest of the molecules.
The condensed structural formula for acetic acid the main component in vinegar is CH3COOH. The chemical formula for acetic acid pictured is C2H4O2. The systematic IUPAC name of acetic acid is ethanoic acid and its chemical formula can also be written as C 2 H 4 O 2.
It is a carboxylic acid consisting of a methyl group that is attached to a carboxyl functional group. The chemical formula for 1-Pentanol is C5H12O. Condensed structural formula of butyl alcohol.
What is the structure of carboxylic acids. Its condensed structure is HCOOH. Structure of Ethanoic Acid.
A HBr b sulfurous acid. Condensed structural formula of propyl alcohol. Carboxylic Acids and Their Salts Acetic Acid Benzoic Acid A1 Condensed structural formulas A2 Solubility in cold water A.
Draw the expanded structural formula for 13-dichlorocyclopentane. For that reason pure acetic acid sometimes called concentrated acetic acid came to be known as glacial acetic acid a name that survives to this day. As their formulas suggest an acetic acid molecule contains 2 carbon atoms 4 hydrogen atoms and 2 oxygent atoms while a molecule of 1-Pentanol comprises 5 carbon atoms 12.
Propanoic acid c 2 h 5 cooh has the structural formula. The condensed formula is CH3CH226CH3. Transcribed image text.
Condensed formula notation simply excludes the lines. Sometimes you will see hoac as an abbreviation. The chemical formula of Ethanoic Acid is CH 3 COOH which can also be written as CH 3 CO 2 H or C 2 H 4 O 2 in the condensed form.
Acetic acid is an organic compound with the formula CH 3 COOH. Molecular formula is AcOH and condensed structural formula is eqrm CH_3COOH eq. Write the acid name or chemical formula for each of the following.
CH 3 CO 2 H. PH A4 Solubility in hot water A5 NaOH A6 HCI Write equations for the reactions of the carboxylic acids with NaOH A5. The third homolog propionic acid CH 3 CH 2 COOH is seldom encountered in everyday life.
Acetic acid also known as ethanoic acid and methanecarboxylic acid is a colorless liquid that has a strong and distinct pungent and sour smell. Module 12 Homework Problem 1415 Write the condensed structural formula of the ester formed when each of the following reacts with methyl alcohol Part A acetic acid Express your answer as a condensed structural formula ΑΣΦ. 1-propanol and acetic acid Condensed Structural Formula.
Find the condensed formula of linolenic acid in Table 151 and use example 151 as a guide. The formula of compounds showing all the atoms excluding most of the bonds in them can be termed as condensed structural formula. Ethanoic Acid freezes during winters and forms a glacier like appearance.
The condensed structural formula for the compound 1-Octanol is C8H18O. Its molecular formula is C 2 H 4 O 2 and its molar mass is 6005 gmol. A chemical reaction does not occur for this question Submit Request Answer Part B butyric acid Express your answer as a.
The final and most common method of writing structures and formulas involves condensed formula notation. Therefore it is also sometimes referred to as glacial acetic acid and is a very common laboratory chemical. Condensed structural formula of isopropyl alcohol.
For that reason pure acetic acid sometimes called concentrated acetic acid came to be known as glacial acetic acid a name that survives to this day. The chemical formula of acetic acid is CH 3 COOH. Condensed structural formula of alcohol and carboxylic acid.
The third homolog propionic acid CH 3 CH 2 COOH is seldom encountered in everyday life. Name the following condensed structural formula of alcohols given below. Condensed structural formula of ethyl alcohol.
Here are their structural formulas instead. Acetic acid CH3COOH or C2H4O2 CID 176 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Acetic anhydride CH3CO2O or C4H6O3 CID 7918 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
Name condensed to the. An expanded structural formula shows all the atoms of the molecule and all the bonds between the atoms in the molecule.
The main difference between light microscope and electron microscope is that beam of electrons is used for magnifying the image of an object while visible light is used in the light microscope to magnify images of tiny areas of materials or biological specimens. The electronic microscope is better than the light microscope because the electronic microscope has more characteristics than the light microscope.

Compound Light Microscope Electron Microscope Microscope Microscope Parts
It is common to have some confusion when distinguishing between optical and electronic microscopes since they have different functionalities.

Electron microscopes vs light microscopes. The light and electron in the names refer to the radiation being used. Electron microscopes have a range of disadvantages as well. The image formed by scattering or transmission of electrons.
Ad Get More Out of Your Digital Microscope and Choose a DSX1000 Model that Suits Your Needs. The image formed by this microscope has a remarkable three-dimensional appearance. Typically magnification of scanning electron microscope is 10 to 500000 times.
What are the advantages and disadvantages of light microscopes and electron microscopes. Light microscopes have high resolution. With an electron microscope you cannot observe live specimens.
Electron microscope vs. However light microscopes are much more practical in general use. They are heavier and larger than light microscopes and operate under a high vacuum.
Uses Electron beam to illuminate the object. It is the main difference between light microscope and electron microscope. In this microscope the image is formed by electrons reflected back from the object.
Electron microscopes are helpful in viewing surface details of a specimen. To enlarge images of elements imperceptible to the naked eye are completely different. The main difference between light and electron microscopes is the radiation used to form an image.
In fact electron microscopes need to be registered with sate environmental regulators. 37 rows Both light microscopes and electron microscopes use radiation light or. There are two fundamental types of microscopes.
The image formed by absorption of light waves. The electron microscope is very complicated and large. And electron microscope which employ electromagnetic lenses and beam of electrons for image formation.
Differences between light microscope and electron microscopes. Both electron and light microscopes form larger and more detailed images of small objects that cannot be formed by the human. One of the characteristic difference is that a light microscope uses a light source whereas an electron microscope uses a beam of an electron.
Following are the differences. Trust Our Long History in Cutting-Edge Optics for Superior Quality Advanced Performance. Both microscopes are used for research and study purposes in biology medical sciences and material.
The main difference between light microscope and electron microscope is that beam of electrons is used for magnifying the image of an object while visible light is used in the light microscope to magnify images of tiny areas of materials or biological specimens. The main difference between light microscope and electron microscope is that light microscopes use beams of light to illuminate the object under examination while the electron microscope uses beams of electrons to illuminate the object. The higher resolution may also give the human eye the subjective impression of a higher depth of field.
Uses light to illuminate the object. List the similarities and differences between electron microscopes and light microscopes. Light microscopes can be used only in the presence of light and are costly.
The specimen must be fixed or stained. However light microscopes form real colour images and can be used to watch living processes occur in microscopic detail while electron microscopes cannot be used to study living cells. Electron microscopes uses short wavelength of electrons and hence.
Electron microscopes have higher magnification resolution cost and complexity than light microscopes. Ad Get More Out of Your Digital Microscope and Choose a DSX1000 Model that Suits Your Needs. In simple terms light microscopes use light which is fairly harmless while electron microscopes put off some ration which is less-so.
Preparing the specimen is more labor intensive generally requires a high skill level and can take a few days to complete. In scanning electron microscopy SEM due to the nature of electrons electron microscopes have a greater depth of field compared to light microscopes. The basic Difference Between Light microscope and Electron microscope is that These two types of microscopes all and having the same objective.
Using visible light as a radiation has several limitations which the electron microscope lessens. Trust Our Long History in Cutting-Edge Optics for Superior Quality Advanced Performance. The light microscope shows low magnifying and resolving power of 1000X and 02µm respectively.
Light Microscope uses a glass lens. They are optical light microscopes which employ glass lenses and visible spectrum of light. The main difference between light microscope and electron microscope is that beam of electrons is used for magnifying the image of an object while visible light is used in the light microscope to magnify images of tiny areas of materials or biological specimens.
An electron microscope utilises electron beams to enlarge an object while a light microscope uses light rays to magnify any object.
What is the symbol for palladium. Elemental hydrogen H element 1 nitrogen N element 7 oxygen O element 8 fluorine F element 9 and chlorine Cl element 17 are all gases at room temperature and are found as diatomic molecules H2 N2 O2 F2 Cl2.

Pin By Richard Vaughn On Chemistry Chemistry Education High School Chemistry Sixth Grade Science
The gaseous element group.

Periodic table gases at room temperature. Ppt The Periodic Table Of Elements Powerpoint Presentation Free. At 0C the elements in their gaseous form are Hydrogen Helium Nitrogen Oxygen Fluorine Neon Chlorine Argon Krypton Xenon and Radon. We can use this Periodic Table of the Elements and find that at a given temperature the elements that are in red are in their gaseous stage.
Hydrogen oxygen nitrogen neon argon helium radon. Hart gave you 118 2. __8762 amu or g_____ 5.
How many elements are gaseous at room 24 periodic table gases at room element is liquid at room temperature classification of the elements. C The freezing point of chlorine is -101C. Click any element below to see all the samples of that element.
How many elements are listed in the periodic table. Elements That Are Liquid At Room Temperature. In the periodic table group 17 Halogen family have metals non-metals liquid and gas at room temperature.
The whole group contains gases those are helium He neon Ne argon Ar krypton Kr xenon Xe and radon Rn. Click here to buy a book photographic periodic table poster card deck or 3D print based on the images you see here. What is the atomic number of selenium.
The noble gases are found in group 18 and exist as isolated atoms. There are a total of 11 elements in the periodic table which are gases at room temperature and standard atmospheric pressure. Iodine is a metal whereas chlorine and bromine are the non-metals.
They are all stable because their outer energy level is filled. The elements that are gases at room temperature are radon Rn xenon Xe krypton Kr argon Ar chlorine Cl neon Ne fluorine F oxygen O nitrogen N helium He and hydrogen H. B The density of chlorine gas at standard temperature and pressure is 317 gL.
Hydrogen H nitogen N oxygen O fluorine F chlorine Cl and noble gases helium He neon Ne argon Ar krypton Kr xenon Xe radon Rn are gases at standard temperature and pressure STP. Fluorides are present in liquid state while chlorine bromine and gaseous are present in the form of gases. Hydrogen H nitrogen N oxygen O fluorine F chlorine Cl helium He neon.
At standard temperature and pressure there are 11 which are gasses. D Chlorine combines with sodium to form table salt. Four other elements are liquids slightly warmer than room temperature.
They are always gases at room temperature. The color of chorine gas is green. They are francium cesium gallium and rubidium all metals.
Written By MacPride Sunday December 23 2018 Add Comment. As the group goes down the density of each atom increases. Considering the periodic table theres first of all one prominent group the noble gases group 8A.
Gases On The Periodic Table At Room Temperature. What is the atomic mass of strontium. Tokyo Electron S Tel Augmented Reality Ar Enhanced.
These elements are gasses at room temperature and pressure. How many elements are gases in the periodic table. D Chlorine combines with sodium to form table salt.
Elemental hydrogen H element 1 nitrogen N element 7 oxygen O element 8 fluorine F element 9 and chlorine Cl element 17 are all gases at room temperature and are found as diatomic molecules H2 N2 O2 F2 Cl2. They do not react with other common elements and do not form bonds in nature. They are colorless odorless and tasteless.
They are mercury a metal and bromine a halogen. Periodic Table Gases At Room Temperature. There are 11 total and you may recognize them if youve looked at different sections of the periodic table which catalogs and organizes chemical elements by their structures and similar properties.
Only two elements on the periodic table are elements at room temperature. Oxygen Hydrogen Nitrogen Fluorine Chlorine and all of the inert gases are gases at room temperature and pressure. 115 rows Elemental hydrogen H element 1 nitrogen N element 7 oxygen O element 8 fluorine.
How are elements that are gases at room temperature designated in this periodic table.
Every compound has its own CHEMICAL FORMULA and its own NAME. 14 disilicon hexabromide Si2Br6.

Answer Predict The Formula For Magnesium Clutch Prep
5 Ag3P silver phosphide.

Magnesium phosphide formula. Magnesium nitride Mg3N2 6. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. Chemical formulas for ionic compounds are called ionic formulas The chemical formula for an ionic compound.
Li 4C lithium carbide Ba 3N 2 barium nitride MgBr 2. For example Cu. The total positive charge must balance the total negative charge.
You should complete this by Sunday. Zinc iodide ZnI2 3. Al 3 Aluminum.
I Magnesium chloride HNO 3 A Nitric acid Ca 3 P 2 I Calcium phosphide ZnCO 3 I Zinc carbonate NaNO 3 I Sodium nitrate H 2 SO 3 A Sulfurous acid AgCl I Silver chloride CH 4 M Carbon tetrahydride Cu 2 C 2 O 4 I Copper I oxalate SnI 4 I Tin IV iodide PbS 2 I Lead IV sulfide CO 2 M Carbon dioxide Al 2 SO 4 3 I Aluminum sulfate. 24304-00-5 AlNO 2 3. P silver phosphide 6 IO 2 iodine dioxide 7 VO 2 vanadium IV oxide 8 PbS lead II sulfide 9 CH 4 methane 10 N 2 O 3 dinitrogen trioxide Write the formulas of the following chemical compounds.
Rubidium and bromine. 3 aluminum oxide Al and O 6 magnesium phosphide Mg and P Formula. The key to writing proper ionic formulas is simple.
Magnesium phosphide Mg 3 P 2 also is moisture sensitive. 2 calcium chloride Ca and Cl 5 sodium nitride Na and N Formula. Ag Silver.
Gallium and oxygen. 5 mercury I phosphide Hg 3P cobalt III phosphide CoP cesium nitride Cs 3N magnesium oxide MgO phosphorus III chloride PC ℓ 3 2. If H is the positive ion the compound is an acid as we will see later on page 6 The positive ion cation may be a monatomic metal ion such as.
Because the charges on the ions are. Copy this to my account. Sodium thiosulfate ____Na2S2O3_____ 3.
11 tetraphosphorus triselenide P4Se3. Use Lewis dot structures to show the covalent bonding in the following pairs of. Chemical formulas for ionic compounds are called ionic formulas.
Aluminum and iodine d. Sodium chloride NaCl and magnesium oxide MgO. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A.
6 IO2 iodine dioxide. 616 Write the symbols for the ions and the correct formula for the a. Again you may be familiar with some of these ions.
Give the formula for the following. A proper ionic formula has a cation and an anion in it. 8 PbS lead II sulfide.
For those with electropositive metals the. The key to writing proper ionic formulas is simple. Name the following binary compounds.
A monatomic meaning one-atom cation takes its name from the name of the element. Lead II chloride PbCl2 5. 12 potassium acetate KC2H3O2.
Sodium and nitrogen c. Write the symbols for the ions and the correct formula for the ionic compound formed by each of the following. CHARGE FORMULA NAME CHARGE FORMULA NAME 1 H Hydrogen 1- H- Hydride Li Lithium F-Fluoride Na Sodium Cl- Chloride K Potassium Br- Bromide Cs Cesium I- Iodide Ag Silver 2 Mg2 Magnesium 2- O2- Oxide Ca2 Calcium S2-Sulfide Sr2 Strontium Se2-Selenide Ba2 Barium Zn2 Zinc Cd2 Cadmium 3 Al3 Aluminum 3- N3- Nitride P3- Phosphide Table 2 Metals That Form More.
Sulfur dioxide ____SO2_____ 2. Na Mg 2 Non. Binary Ionic Compounds Type II The cation of a transition metal is always named first like any cation and the anion second.
An ionic compound is never formed between two cations only or two anions only. Magnesium Mg 2 mercury I Hg 2 2 nickel Ni 2 potassium K rubidium Rb silver Ag sodium Na strontium Sr 2 tin II Sn 2 zinc Zn 2 Examples of Negative Ions. Iron III oxide Fe2O3 4.
An ionic compound is never formed between two cations only or two anions only. Hg23P2 mercury I phosphide Section D Write the formula for the ionic compounds containing transition metals BE CAREFUL TRANSITION METALS MAY HAVE ROMAN NUMERALS and NICKNAMES 1. These species are insoluble in all solvents - they are 3-dimensional solid state polymers.
Superscripts are used to. Use the Stock system where necessary. The transfer of electrons between metals and non-metals produces charged particles called ions.
2 provides a dispersion formula based on data from ref. Just as atoms can lose electrons to become cations some can gain electrons and become negatively charged anions. Complete these in lab and on your own time for practice.
10 N2O3 dinitrogen trioxide. Copper phosphide Cu 3 P illustrates a rare stoichiometry for a phosphide. The nomenclature naming systems for IONIC and MOLECULAR compounds are different.
7 VO2 vanadium IV oxide. Calcium and chlorine b. Potassium and sulfur b.
Use the stock form for the transition metals. Fluoride is sometimes added to community water supplies. Ca 2 Calcium.
Optical constants of CaCO 3 Calcium carbonate Calcite Ghosh 1999. Predicting the Formula of an Ionic Compound In a chemical formula subcripts are used to specify the numbers of a type of atom in the formula. A proper ionic formula has a cation and an anion in it.
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. E-mail to a friend. Binary Ionic Formula Practice Name_____ 1 sodium chloride Na1 Cl-1 NaCl 2 lithium bromide Li1 Br-1 LiBr 3 magnesium flouride Mg2 F-1 MgF2 4 potassium oxide K1 O-2 K2O 5 calcium sulfide Ca2 S-2 CaS 6 aluminum iodide Al3 I-1 AlI3 7 barium bromide Ba2 Br-1 BaBr2 8 aluminum sulfide Al3 S-2 Al2S 3 9 calcium phosphide P-3 Ca2 P-3.
11 tetraphosphorus triselenide P 4 Se 3 12 potassium acetate KC 2 H 3 O 2 13 iron II phosphide Fe 3 P 2 14 disilicon hexabromide Si 2 Br 6. 1 barium oxide Ba and O 4 sodium oxide Na and O Formula. Indium phosphide InP and gallium phosphide GaP are used as a semi-conductors often in combination of related arsenides.
Formula 21 sodium phosphide Na 3 P 22 magnesium nitrate MgNO 3 2 23 lead II sulfite PbSO 3 24 calcium phosphate Ca 3 PO 4 3 25 ammonium sulfate NH 4 2 SO 4 26 silver cyanide AgCN 27 aluminum sulfide Al 2 S 3 28 beryllium chloride BeCl 2 29 copper I arsenide Cu 3. 13 iron II phosphide Fe3P2. Data Expressions for n CSV - comma separated TXT - tab separated Full database record Optical transmission calculator.
Metals lose electrons to produce positve ions called cations. 4 copper I phosphide 5 tin IV selenide 6 gallium arsenide 7 lead IV sulfate 8 beryllium bicarbonate 9 manganese III sulfite 10 aluminum cyanide 11 CrPO 4 2 12 VCO 3 2 13 SnNO 2 2 14 Co 2 O 3 15 TiC 2 H 3 O 2 2 16 V 2 S 5 17 CrOH 3 18 LiI 19 Pb 3 N 2 20 AgBr 21 NaBr sodium bromide 22 ScOH 3 scandium III. Iron II fluoride FeF2.
Chemical Formula Nomenclature Practice. These consist of any positive ion except H combined with any negative ion. The total positive charge must balance the total negative.
An ionic compound is composed of a metal and a non-metal. Formula Type Chemical Name HCl A Hydrochloric Acid Hg 2 SO 4 I. Cadmium sulfide CdS 2.
Write the correct chemical formula for the ionic compound that forms. For example O 2 is interpreted as a molecule formed by two oxygen atoms and CH 3 OH is interpreted as a molecule with one carbon four hydrogens and one oxygen. Ionic Compound Naming and Formula Writing List 1.
Write the formulas of the following chemical compounds.
Preparation of alkyl halides from alcohols. Formation of nitrate esters.

Dibal H Aldehydes And Ketones Organic Chem Chemistry Ochem
C 4 H 6 OH 4.

Examples of alcohols in organic chemistry. Reactions of alcohols involve oxidations substitutions and eliminations giving you a significant advantage in synthesis and functional group modifications. C 2 H 4 OH 2. This is usually manifested as an increase.
C 7 H 9 OH 7. This is the currently selected item. The boiling point of ethanol for example is 785ºC whereas propane with about the same molecular weight boils at -421ºC.
The alcoholic functional group is the OH or hydroxyl group attached to a carbon atom. In this method an alcohol is reacted with a carboxylic acid in the presence of an inorganic acid catalyst. Naming Alcohols with Practice Problems.
Alcohol contains one or more hydroxyl groups directly attached to the carbon atom of an aliphatic chain. When the hydroxyl is attached to a carbon atom in an aromatic system it is known as phenol. CH3CH2CH2OH H2SO4 140C H2SO4 140C CH3CHCH3 OH H2SO4 180C CH3CHCH2CH3 OH 32 Oxidation of Alcohols to Carbonyl Compounds An oxidation reaction occurs when a molecule loses electrons.
Methanol is used as a petrol additive in the UK to improve combustion of petrol. The Grignard reaction is the only simple method available that is capable of producing primary secondary and tertiary alcohols. Alcohol Reactions in Organic Chemistry and Synthesis.
Alcohols can be converted to esters by means of the Fischer Esterification Process. Science Organic chemistry Alcohols ethers epoxides sulfides Alcohol nomenclature and properties. Science Organic chemistry Alcohols ethers epoxides.
An example is the reduction of methyl benzoate to benzyl alcohol and methanol. Identify the parent chain. Alcohols containing two or more hydroxyl groups can be made.
Examples include 12-ethanediol ethylene glycol used in antifreeze and 123-propanetriol glycerine used as a solvent for cosmetics and medicines. 74 Acid-Catalyzed Dehydration of Alcohols Rearrangements also known as 12 shifts can occur in primary and secondary alcohol dehydration The more favored product is dictated by the stability of the alkene being formed orF dehydration of secondary alcohols the positive charge is shifted through a hydride shift or alkyl shift. Number the parent chain giving the.
Ethanol is a primary alcohol because there is only one alkyl group attached to the carbon that carries the OH substituent. Alcohol function is an extremely versatile functional group in organic chemistry. Leaving groupIn organic chemistry the species that leaves the parent molecule following a substitution reaction.
SN1 and SN2 reactions of alcohols. Grignard reaction with aldehydes and ketones. C 6 H 8 OH 6.
Alcohols are an important class of molecules with. Naming alcohols follows the same rules we discussed earlier for the IUPAC nomenclature rules for alkanes. HCl is the weakest acid among these and Cl is not a strong nucleophile so the reaction with HCl.
Chapter 3 Alcohols Phenols and Ethers 16 31 Examples. Alcohols can form similar hydrogen bonds as shown in the figure below. Combustion of alcohols Alcohols such as ethanol and methanol are used as fuels making use of combustion.
Methyl and primary alcohols are converted to alkyl halides via S N 2 reaction according to the general mechanism shown below. As a result alcohols have boiling points that are much higher than alkanes with similar molecular weights. Reactions of alcohols is a typical topic in a sophomore organic chemistry and is covered in either first or.
It also has increasing importance as a feedstock in the production of organic chemicals. Ethanol is used as a petrol substitute in countries with limited oil reserves. In a tertiary 3 alcohol the carbon atom holding the -OH group is attached directly to three alkyl groups which may be any combination of same or.
The I and Br are strong enough nucleophiles to attack the primary carbon and the OH 2 in turn is an excellent leaving group in form of neutral water molecule. Preparation of mesylates and tosylates. Because the reaction is an equilibrium reaction in order to receive a good.
Alcohols are organic compounds in which the hydroxyl functional group -OH is bound to a carbon atom. This is the brief summary of steps. Dehydration of Alcohols Complete the following reactions.
As we noted in Chapter 4 Covalent Bonding and Simple Molecular Compounds Section 46 Introduction to Organic Chemistry methanol CH 3 OH and ethanol CH 3 CH 2 OH are the first two members of the homologous series of alcohols. In a secondary 2 alcohol the carbon with the -OH group attached is joined directly to two alkyl groups which may be the same or different. C 5 H 7 OH 5.
Oxidation of alcohols II. C 3 H 5 OH 3. C 3 H 6 OH 2.
To produce a primary alcohol the Grignard reagent is reacted with formaldehyde. The isopropyl alcohol found in rubbing alcohol is a secondary alcohol An example of a tertiary alcohol is tert-butyl or t-butyl alcohol or 2-methyl-2-propanol. The name of an alcohol comes from the hydrocarbon from which it.
The chemical formula for table salt is NaCl. What is the chemical formula for salt.

Product Name Penicillin V D5 Potassium Salt Phenyl D5 Pharmaffiliates Penicillin Potassium Salt
The most common salt that is relevant to our daily lives is table salt which is made up from a Na and a Cl- making its chemical formula NaCl.

What is salt chemical formula. Chemical Formula For Sugar 9. You might know this substance as table salt and it is NaCl. Magnesium sulfate is an inorganic salt with the chemical formula MgSO4H2Oxwhere x varies from 0 to 7.
Since both charges are 1 we drop both numbers and we see that the salt is composed of one lithium atom and one bromine atom. The chemical formula of the salt depends on the acid which represents the source of the anion the valence of the anion and action. The chemical formula of Epsom salt is Magnesium sulfate.
What is the chemical formula of hydrated Epsom salt. Sir Humpty Davy was the chemist who separated the salt in sodium and chloride for the first time in 1807. Salt chemical formula.
Chemical Composition Of Chaka Salt Lake Water River And. Anhydrous monohydrated and heptahydrated form and its chemical formula are. Salt chemical formula.
Ssd solution chemical formula. It is often encountered as the heptahydrate sulfate mineral epsomite MgSO47H2O commonly called Epsom salt. Magnesium sulfate also known as Epsom salt is an inorganic salt mainly used in agriculture as fertilizer.
What is the chemical formula for salt. It is also used by the pharmaceutical industry. Heptahydrate sulphate mineral epsomite MgSO47H2O.
The scientific name for salt is sodium chloride. Table salt molecular formula sodium chloride table salt chemical formula structured. Since table salt is an ionic compound the formula implies that numbers of Na ions and Cl-.
What is ssd solution formula. What is the chemical formula of salt. You must login to add an answer.
For non-molecular substances such as table salt we represent the composition with an empirical formula. Sodium chloride is represented by NaCl meaning that sodium and chlorine ratio in sodium chloride is 1 to 1. Magnesium sulfate has three known form.
Magnesium sulfate chemical formula MgO4S and CAS No. FeCl 3 is the named as iron III chloride while AlCl 3 is aluminum chloride although the valence of iron and aluminum is 3 in the two salts because iron has two valencies II III while aluminum has only. If you are talking about common salt then its formula is NaCl.
If you are talking about common salt then its formula is NaCl. Chemical formula SaltWhat is the chemical formula for saltFacebook. Thoughts Flow For You.
MgSO 4 MgSO 4 H 2 O and MgSO 4 7H 2 O and the molar mass are 120361 g mol -1 138361 g mol -1 and. Common table salt is also called sodium chloride and the chemical formula for salt is Na Cl. Its chemical formula is NaCl.
Basically when we listen salt it may be sea salt also which contains many chemicals but the salt which is used in households is sodium chloride and the chemical formula for this salt is NaCl. What is the chemical formula of salt. Salt is an ionic compound made up of sodium and chloride ions.
7487-88-9 is an inorganic salt chemical compound containing magnesium sulfur and oxygen. Salt consists of one atom of sodium combined with one atom of chlorine. Taking the table salt eqNaCl eq as an example the salt molecule is.
Solved Equation Writing Translate Each Of The Follow Che. The chemical formula for salt is NaCl. Why sodium chemical formula is Na.
In order to derive any salt chemical formula the cation and the anion must first be identified. Some examples of other salts include KCl potassium chloride. Sodium Chloride I Ruishun Trade Co Ltd.
How to get the SSD solution chemical formula. Also called sodium chloride natrium chloride or halite table salt is an ionic compound that contains a positively charged ion of sodium and a negatively charged chloride ion connected through an ionic bond. It can have impurities of gypsum CaSO4 and sylvite KCl but it is very rare to find potassium sulfate as a mineral although occasionally polyhalite K2Ca2MgSO442H2O is It is typically formed by the evaporation of salty water such as sea water.
Sodium chloride commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a. Salt is a thing that we sprinkle on our food give it a taste. What is salt chemical formula.
The chemical Mohrs salt formula of the double salt is FeSO 4NH 4 2 SO 46H 2 O Ferrous Ammonium Sulfate Hexa Hydrate or NH 4 2 FeSO 4 26H 2 O Ammonium IronII SulphateIts name derived from the name of German Chemist Karl Friedrich Mohr who proposed various important approaches of titration in the 19th century. Diy Chemical Formula Salt And Pepper Shaker Set Divas. This is the common name for the mineral halite.
It is clear from the table salt chemical formula that it contains two elements ie. Again the subscript 1 is omitted. The formula H2O is also the molecular formula of water.
The suffix of the name reflects the type s of functional group s. It contains examples with substituents cycloalkenes.

Saat On Twitter Important Revision Of Organic Compounds Standardtest تحصيلي تحصيلي كيمياء S In 2021 Organic Chemistry Teaching Chemistry Organic Chemistry Study
1 1 2-Epoxy propane 2 Propylene oxide 3 1 2Oxo propane 4 1 2Propoxide.

Organic chemistry iupac naming practice. When naming organic compounds there are strict rules regarding punctuation. IUPAC name is 22- dimethyl propane. This video is a MUST for breaking down.
Organic chemistry naming examples 2. The examples cover the nomenclature of alkanes bicyclic compounds alkenes alkynes alcohols alkyl halides aromatic compounds aldehydes and ketones amines ethers and carboxylic acid derivatives such as nitriles esters and. The first video shows you how to break down the name of an organic molecule using my puzzle piece approach.
Play this game to review Organic Chemistry. The IUPAC name of the compound is. IUPAC Nomenclature Practice Problems - Chemistry Steps.
It provides an unambiguous structure. Use numbers commas dashes and spaces in your names wherever appropriate. Complete your research with top quality products.
Preview this quiz on Quizizz. What is the organic. This is a set of practice problems on naming organic compounds.
A hyphen is used to sep. State the correct IUPAC name of the following hydrocarbons. For naming purposes the functional groups are assigned with priorities Table 23.
A comma is used to separate two numbers. The syntax required is very specific. Solved Jun 16 2020.
Ad Enabling you to solve the toughest problems in life science. A systematic method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry IUPAC. These names are listed within the discussion of naming alkanes.
A simplified version Introductory IUPAC Organic Nomenclature is also available for high school andor college or. 80 thoughts on Organic Chemistry IUPAC nomenclature Practice Sheet with answers. Hence option 4 is the answer.
In general the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. Version 30 Updated 2014. If the compound includes more than one functional groups the one with the highest priority is the parent structure and determines the parent name.
The other groups will be regarded as substituents. Official IUPAC naming recommendations are not always followed in practice and the common or trivial name may be used. In order to name organic compounds you must first memorize a few basic names.
Some of the worksheets displayed are naming organic compounds practice practice 8 1 give the iupac name of each of the following naming organic compounds bcpldtpbc note 201306 acc j naming hydrocarbons work and key nomenclature in organic chemistry chemistry 1a nomenclature. Preferred names in the nomenclature of organic compounds. Nomenclature of Inorganic Chemistry.
Suffix is used to indicate the name of the parent structure and prefix is for the substituent. Naming alkanes cycloalkanes benzene derivatives. To review hydrocarbon nomenclature and see some examples click the button below.
Organic chemistry naming examples 4. Extension and Revision of the Nomenclature for Spiro Compounds Including Bicyclic Compounds IUPAC Recommendations 1999. These materials provide a step-by-step guide to learning organic nomenclature and are intended for those taking Introductory Organic Chemistry at a college or university.
The following guidelines for organic nomenclature are based on the def. Draft 7 October 2004. Complete your research with top quality products.
For naming organic compounds - some have had limit scope or become embedded in common us age and some have persisted over time The International Union of Pure and Applied Chemistry IUPAC periodically reviews naming practice attempting to standardise nomenclature. Name the unsaturated compounds. Hence option 1 is the answer.
Assign A Systematic Iupac Name To Each Of The Following Aldehydes And Ketones Practice Problems Ketones Chemistry Organic Chemistry. What is IUPAC nomenclature. Organic Chemistry Reactions 10th Higher Ed Worksheet Chemistry Worksheets Organic Chemistry Reactions Organic Chemistry.
The rules for naming organic compounds are tedious and can become overwhelming fast. Provide the IUPAC name for this 2-methylbutyl-substituted alkane. March 1 2014 at 100 pm.
Naming two isobutyl groups systematically. Video 1 Introduction To IUPAC. Organic chemistry naming examples 3.
Ad Enabling you to solve the toughest problems in life science. Your sample paper for nomenclature practice sheet for class xi cbse shall i have your solution this respect. Naming Organic Compounds Worksheet.
The IUPAC name of CH 3 COCHCH 3 2 is 1 2-methyl-3-butanone 2 4-methylisopropyl ketone 3 3-methyl-2-butanone. This organic chemistry video tutorial explains how to name alkenes using the iupac nomenclature system.
We report a rod-like sulfonated viologen R-Vi anolyte for aqueous redox flow batteries AORFBs. The COF nanosheets had a BET surface area of 30 cm 2 g which were consistent with the reported values.

Designer Two Electron Storage Viologen Anolyte Materials For Neutral Aqueous Organic Redox Flow Batteries Sciencedirect
Yu Liu Qiang Li Dong-Sheng Guo and Kun Chen Supramolecular chain-like aggregates and polymeric sandwich complexes constructed from p-sulfonatocalix46arenes with 8-hydroxyquinoline guests PDF 2008 106 675.

Methyl viologen solvent. Opiri Abdulrahman Ezzir Guoqiang Li and. R-Vi possesses a low membrane-penetration rate of 10 10 cm 2 s 1 and the lowest redox potential of 055 V vs. A flow battery or redox flow battery after reductionoxidation is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane.
Switchable solvent-controlled divergent synthesis. On adding 05 equivalent of CB8 to a solution of TMV 2 guest the fluorescent colors change from blue λ max 460 nm to yellow λ max 535 nm. Peng Liang Heng-Yi Zhang Zhi-Lin Yu and Yu Liu Solvent-Controlled Photoinduced Electron Transfer between Porphyrin and Carbon Nanotubes PDF 2008 736 2163-2168.
Recycling Thermoset Epoxy Resin Using Alkyl-Methyl-Imidazolium Ionic Liquids as Green Solvents. An efficient and regioselective approach to pyrimidine and dibenzobf14oxazepine derivatives Org. IF 5281 Pub Date.
Among the redox active molecules methyl viologen MV is the most promising redox species in an aqueous organic redox flow battery featuring the stable redox reaction MV 2 MV. Control experiments confirmed that BCN NiCl 2 dppp and visible light were all important in our system as rather low conversions were observed in the absence of those reaction components Table 1. Ma and co-workers have synthesized a color-tunable host-guest complex which is composed of thiazolothiazole methyl viologen TMV 2 guests and CB8.
SHE among one-electron-storage viologens in AORFBs simultaneously. For the different redox states of methyl viologen c 10 mol dm 3 was applied but for the πdimer and σdimer states c 05 mol dm 3 concentration was considered. Hopkins WHuner N-Introduction to plant physiology-2008pdf.
2019-11-20 Jin-Peng Meng Wan-Wan Wang Ying-Lu Chen Saurav Bera Jun Wu 更新日期2019-11-20 详情 收藏 Transition-metal-free direct C-3 cyanation of quinoxalin-21H-ones with ammonium thiocyanate. Ion exchange accompanied by flow of electric current occurs through the membrane while both liquids circulate in their own respective space. The viologen-iodide viol-I polypeptide 6-I was obtained via S N 2 substitution of halide-containing side chains by the viologen precursor 1-methyl-44bipyridylium iodide MBPI.
The intercalation potentials were evaluated by CV and galvanostatic experiments in a non-aqueous system using a mix of solvent. The ICSC project is a common undertaking between the World Health Organization WHO and. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
Stable Diarylamine Substituted Tris246-trichloro-phenylmethyl Radicals. 5 Twelve-connected Net with Face-centered Cubic Topology. Warner ACS Applied Polymer Materials 2021 3 11 5588-5595 Article ACS Editors Choice.
6 Solvent Control in the Hydrothermal Synthesis of Two CopperI Iodide-benzimidazole Coordination Polymers Wu. Calculate the molecular mass the elementary analysis and the molecular mass distribution spectrum of a molecule based on the isotopic composition of the chemical elements. The enthalpic hydrophobic effect and entropic hydrophobic effect.
3 16 17. The hydrophobic effect is caused by the exclusion of nonpolar moieties from an aqueous environment and which drives the aggregation of these nonpolar solutes. Direct measurement of excited-state lifetimes by femtosecond fluorescence up-conversion.
Solvent reorganization controls the rate of proton transfer from neat alcohol solvents to singlet diphenylcarbene. A Coordination Polymer Based on Cu 12 μ 4 -SCH 3 6 6 Clusters and CN-. Journal of the American Chemical Society.
Its electrochemical properties and solubility significantly depend on the. Other competitive redox couples have been investigated contributing to the high voltage and energy density of ABs like negatively charged couples of FeCN 6 4 FeCN 6 3 I 3 I and Br 3 Br to suppress OER on the cathode and positively charged couples of MV 2 MV and HV 2 HV MV and HV refer to methyl viologen and heptyl viologen respectively to replace. DNARNA nucleotides and nucleosides.
The main target users are workers and those responsible for occupational safety and health. Calculate the global 3D structure of a double-stranded DNA molecule from its nucleotide sequence according to the dinucleotide wedge model. CrystEngcomm 2005 7 514-518.
1315 Two energetic components comprise the hydrophobic effect. 3 12 It has been widely studied due to the significant role it plays in chemistry and biology. The R-Vi-based AORFB exhibits an extremely low capacity-fade rate of 0007 per cycle in 3200 continuous.
Trace amount of product was detected using H 2 as the hydrogen source indicating a transfer hydrogenation process from protic solvent instead of direct hydrogenation Table 1 entry 6. Further treatment under ultrasonication in solvent generated viologen-COF nanosheets with sizes in a range of 100300 nm and a thickness of 5 nm as determined from the TEM and AFM images in Figures S2-S3. One-Step Synthesis Near-Infrared Emission and Redox Chemistry Chuan Yan Dongyue An.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
C 3 H 6 O 3. Shop now at NET-A-PORTER.
We are unable to retrieve the vendor information for this entry at this time.

Structure of lactic acid. Lactic acid is an alpha hydroxy acid whose formula is. It consists of an acid functional group -COOH and a hydroxyl group -OH. It has two optical isomers Levo and Dextro making itself a chiral molecule.
Did you know that active people or even a more fidgety person produces more CO2 and Lactic Acid on a daily basis. Structure and Morphology of Polylactic acid Stereocomplex Nano-fiber Shish Kebabs Qing Xie12 Xiaohua Chang12 Qian Qian2 Pengju Pan1 Christopher Y. L--lactic acid Lactic acid 79-33-4.
Lactic acid has a formula of C 3. Rm CH_3CH OHCOOH CH3. You can also browse global suppliersvendorpricesPricemanufacturers of Lactic acid 50-21-5.
Lactic Acid in its most basic form is a build up of chemical compounds in muscle tissue. Lactic acid is a compound formed as a result of energy-producing processes in your body as well as one thats formed on an industrial scale for many applications. When milk sugar lactose undergoes fermentation the product obtained is lactic acid.
The chemical formula of Lactic Acid is rmC_rm3rmH_rm6rmO_rm3rm Its IUPAC name is 2-Hydroxypropanoic acid. Lactic Acid was first discovered in the 1780s by a Swedish chemist named Carl Wilhelm Scheele. This entity has been manually annotated by the ChEBI Team.
C H 3 C H O H C O O H. L-isomers are commonly present among living organisms. This reaction in addition to producing lactic acid also produces nicotinamide adenine dinucleotide NAD that is then used in glycolysis to produce energy source adenosine triphosphate ATP.
Lactic acid is one of the organic acids. These components are not identical because they cannot be superimposed on each other. The lactic acid has a significant part in various biochemical processes.
It is also known as milk acid. Lactic Acid Chemical Formula and Structure. What is Lactic Acid.
Ad Discover the power of beauty. Ad Discover the power of beauty. It has a molecular weight of 90080 gmol and is classified as an alpha-hydroxy acid.
Lactic Acid is an organic acid with chemical formula C 3 H 6 O 3. Lactic Acid DL- is the racemic isomer of lactic acid the biologically active isoform in humans. Lactic acid or lactate is produced during fermentation from pyruvate by lactate dehydrogenase.
An optically active form of lactic acid having S -configuration. At lastLactic acid 50-21-5. Lactic acid 2-hydroxypropanoic acid has the formula C 3 H 6 O 3The molecular structure contains both a terminal carboxylic acid and a hydroxyl group in the 2nd position.
Shop now at NET-A-PORTER. It contains three carbon atoms a hydroxyl group and carboxylic acid as a functional group. The optimal biological activity of lactic acid is.
Asked Jul 6 2020 in Chemistry by Aalaya 476k points icse. Name the type of isomerism exhibited by lactic acid CH3CHOHCOOH giving a reason for your answer. The hydroxyl group is attached to the carbon atom that is adjacent to the carboxyl group.
Structure properties spectra suppliers and links for. This reaction in addition to producing lactic acid also produces nicotinamide adenine dinucleotide NAD that is then used in glycolysis to produce energy source adenosine triphosphate ATP. Lactic Acid L- is the levorotatory isomer of lactic acid the biologically active isoform in humans.
Lactic acid has a carbon atom from which a hydroxyl group -OH and a carboxylic group -COOH is joined along with a methyl group -CH3. He isolated the Lactic Acid present in sour milk. Though it contains hydroxyl functional group it exhibits more acidic properties.
Visit ChemicalBook To find more Lactic acid 50-21-5 information like chemical propertiesStructuremelting pointboiling pointdensitymolecular formulamolecular weight physical propertiestoxicity informationcustoms codes. Lactic acid 2-Hydroxypropanoic acid is an example of a compound which shows optical isomerism It contains one asymmetric carbon atom. One is the mirror image of the other.
The orientation of the hydroxyl group allows for two optical isomers l- or S lactic acid and d- or R lactic acidChemical routes typically produce racemic lactic acid while biological routes commonly approach. Two 3 dimensional structures are possible for Lactic acid. It is found in cottage cheese leban sour milk yogurt and Koumiss.
The IUPAC name of lactic acid is 2-hydroxypropanoic acid. Lactic acid or lactate is produced during fermentation from pyruvate by lactate dehydrogenase. Li2 1 State Key Laboratory of Chemical Engineering College of Chemical and Biological Engineering Zhejiang University 38 Zheda Road Hangzhou 310027 China 2 Department of Materials Science and Engineering Drexel University.
The chemical formula of the lactic acid is C3H6O3. The structure of lactic acid is shown in Fig. It contains an asymmetric carbon atom due to which lactic acid exists in two forms as L lactic acid and D-lactic acid.
Chemical Structure of Lactic Acid.
Propene is an organic compound. It is a colorless gas at room temperature.

Table Containing The Molecular Formula Structural Formula And Ball And Stick Model Of Ethene Propene But 1 Ene And But 2 Ene Gcse Chemistry Chemistry Gcse
C 3 H 8.

Chemical formula for propene. Using PV nRT. The molecular weight of propene is about 42081 gmol. CH2 is the empirical formula of propene C3H6.
Propene C_3H_6 is a hydrocarbon which is the term used to designate a compound that consists of only carbon and hydrogen. Going by the formula the propene is called a molecular formula the empirical formula will be. 1-Propene - Physico-chemical Properties.
Chemical Formula of Propane. Since it is only made of hydrogen and carbon atoms it is a hydrocarbon. When a hydrocarbon undergoes complete combustion only two products are formed.
C 3 H 6. A formula that shows the simplest whole number ratio of the elements in a compound eg. C 3 H 6 cyclopropane molecular mass420 and n 3 that is C 1 3 H.
C 3 H 6 propene or propylene molecular mass420 and n 3 that is C 1 3 H. Propylene propene organic matter class alkenes consisting of three atoms of carbon and six atoms of hydrogen. The chemical formula of propane is C3H8 whereas the chemical formula of propene is C3H6.
Molecular Model Application loaded. What is the empirical formula for propene. The chemical formula for propane is C3H8.
The substance is also known as propylene and has the formula C 3 H 6. The chemical formula of propylene is C3H6 H2CCHCH3 rational formula structural. CH 3 CH 2 CH 2.
Propylene also called propene a colourless flammable gaseous hydrocarbon C3H6 obtained from petroleum. Propylene propene formula gas features. Large quantities of propylene are used in the manufacture of resins fibres and elastomers see polyolefin and numerous other chemical products.
The molecular weight of propene is about 42081 gmol. The propane molecule has three carbon atoms and eight hydrogen atoms C3H8 from which a general chemical formula can be derived. Aromatic molecules which contain at least one ring in their structure.
This tells you that all the carbon that was a part of the hydrocarbon will now be a part of the carbon dioxide and all the hydrogen that. Propylene has a double carbon-carbon bond and therefore relates to unsaturated or unsaturated hydrocarbon. Propylene CH3CHCH2 or CH2CHCH3 or C3H6 CID 8252 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
At room temperature and normal pressure it is a gas. There are three types of hydrocarbon molecules. Propene is produced from fossil fuels and from coal.
Empirical Formula is the simplest ratio in a compound. P pressure atm V volume Liters n number of moles mol R gas law constant 00821 LatmmolK T temperature K At STP standard temperature and pressure temperature of a gas is 273K while its pressure is 1 atm. N 1 1174 00821 273 n 1174 2241.
Apart from being used in LPG propane is also used as biofuel in buses and taxis as it is a clean fuel. It is the second-simplest alkene. Propene is an organic compound having the chemical formula C 3 H 6.
There is no double bond in propane while there is a double bond present between the first and the second carbon atom in propene. Propane is an odorless and colorless gas and has several uses. It is separated in large quantities from natural gas light crude oil and oil-refinery propane chemical compound Britannica.
Using the general gas equation formula. 9003-27-4 - Polyisobutylene NF - Similar structures search synonyms formulas resource links and other chemical information.
Add to Wish List. Remember to register your EuroBonus membership number to each booking to earn points and maximize your benefits.
Silver Price Forecast Heading Back To August Low Levels For Xag Usd
Technical momentum remains bullish with silver prices having survived a retest of.

Low silver levels. You can order. However there are different conditions other than diabetes that can also cause blood sugar levels to drop to dangerous levels in cats. 1986 - 2021 Flag Core Silver Eagle Date Run Continuity Program.
Silver XAGUSD licks its wounds near the lowest levels since mid-October during the early Asian session on Tuesday. Shampoos pH Levels Database Verified from MSDS By Camelia Smith Published 28 Jul 2019 Updated 25 May 2021. 1 Troy Ounce 3110 Gram Silver Price Per 1 Gram 075 USD 1 Troy Ounce 0031 Kilogram.
However patients do not always have. We price our products off the silver spot price using the most up to date and accurate data feed. Causes of low blood sugar levels after eating leg pain.
That said the quote eyes to regain 2300 by the press time. What Our Customers Say About Us. Erin Walter meteorologist at the National Weather Service in Grand Junction said the services station located northeast of downtown Steamboat Springs has recorded only 84 inches of snow so far for the 2021-22 winter season.
The market sentiment has slightly improved though it failed to lift silver prospects which fell to a seven-week low. Causes of low blood sugar levels after eating korean natural cure. During the winter mist the plants frequently to help prevent the leaf tips from turning brown.
The move has now stalled and this comes ahead of the next support at 23oz. The machines remove invasive milfoil from. 20 is Opening Day at Steamboat Resort but low snow levels could force the resort to push that date back.
The more you travel during your personal 12-month qualification period the faster you reach the next level and enjoy more benefits. Hence such silver buyers usually prefer less expensive and low-risk silver physical bullion products with lower premiums over spot. Proof and Mint Sets.
In most animals hypoglycemia is actually not a disease in and of itself but is only an indication of another. LOUIS Low water levels on the Mississippi River have exposed the USS Inaugural near downtown St. We estimate that this change will affect up to 72 of CVSs 94 Million covered lives or 68 Million people who have Type I or Type II diabetes as they require daily blood sugar monitoring.
Add to Wish List. Dental amalgam is a mixture of metals consisting of liquid elemental mercury and a powdered alloy composed of silver tin and copper. 1968-2009 Proof Sets in Mint Packaging.
Silver posted a low above 23 last week and on that it is now starting to make good on forming out the right side of an inverse head-and-shoulders pattern. Today the price has risen half a percent but this largely looks like a retracement from the move lower in recent sessions. We would like to show you a description here but the site wont allow us.
The spot price of silver is the basis for determining pricing when you want to buy silver bullion. Rototillers hitting Okanagan Lake for the season this week had to be lifted into the water with cranes due to low lake levels. You always start as a Member.
EuroBonus has 4 membership levels. Kitco News - The price of silver didnt last long above 25oz and has fallen to hit 2362oz. That said the.
LouisThe USS Inaugural a World War II minesweeper used to be a National Historic. Approximately half 50 of. With only two bars the failure of one.
In 2010 just 10000 of these coins were produced. Road traffic is only one source of low-level pollution but is the easy hit for trying to reduce levels. Low serum magnesium levels can result in serious adverse events including muscle spasm tetany irregular heartbeat arrhythmias and convulsions seizures.
The production of silver Libertads has lagged behind the levels of its North American counterpart coins making it a somewhat difficult coin to obtain for some collectors. Financial advisers will usually recommend this strategy as a way to hedge against inflation and a good method of balancing portfolios. A clean daily close above 2486 will have the neckline of the pattern broken and confirmed.
When youre looking at the Mexican Libertad most coins you will find that are. By far it is this test strip switch that will affect the most people. Heres a list of shampoos and hair products pH levels weve put together during our research to help you in case you cant find pH information on your products labels.
Looking at the depth of the pattern the projected target based on measured moves is up around 28. Conversion Silver PriceSpot Price. 2016 Mercury Dime Centennial Gold Coin - SP70 1st Strike.
The US Dollar Index advances 032 sitting at 9639 following the US 10. Low light or indirect light. Available in dark green and variegated forms spider plant Chlorophytum comosum makes a great tabletop or basket plant in low-light conditions.
Up to 12 inches tall. As an Amazon Associate we may earn from qualifying purchases. You can view the current silver spot price at the top of every page on our website.
It is also very lucrative in generating charges and fines income for London. They offer a modest appreciation over time granting them with inflation-proof financial protection. When the soil is dry to the touch.
Silver Price Outlook. Silver spot price is live and is updated on our website every minute the global markets are open. World Coins Currency.
Member Silver Gold and Diamond. Silver posted a low above 23 last week and on that it is now starting to make good on forming out the right side of an inverse head-and-shoulders pattern. In 1999 the National Mint of Mexico only produced 600 1 ounce proof coins.
Get iShares Silver Trust SLVNYSE Arca real-time stock quotes news price and financial information from CNBC. The medical term for critically low levels of sugar in the blood is hypoglycemia and it is often linked to diabetes and an overdose of insulin. Silver Bridge structure Low redundancy high strength The eyebars in the Silver Bridge were not redundant as links were composed of only two bars each of high-strength steel more than twice the tensile strength of common mild steel rather than a thick stack of thinner bars of modest material strength combed together as is usual for redundancy.
Silver prices are benefiting from strong economic data and record low real interest rates.
Fluorides are compounds that. Chemical Formula of Fluorine Gas.

Fluorine Made By Zinaidova D Kuznetsova
The name fluorspar is derived from the Latin fluere to flow.

Fluorine gas chemical formula. What is the Formula for Fluorine Molecule. Calcium fluoride CaF2 in the solid state s A chemical reaction features the reactants on the left side of the reaction arrow and the products on the right side of the reaction arrow. Each bubble of sulfur dioxide gas led into a container of fluorine produces an explosion Mellor 211946-1947.
The mineral subsequently proved to be a source of the element which was accordingly named fluorine. 2F 2 2H 2 O 4HF O 2. Fluoride appears to bind to calcium ions in the hydroxyapatite of surface tooth enamel preventing corrosion of tooth enamel by acids.
Fluorine is a chemical element with an atomic number 9 and is denoted by F. Step 1- The symbol of the positive atom or basic radical is placed on the left -hand side and the symbol of the negative atom or acidic radical is placed on. Oxygen atoms in the water molecules are oxidized to oxygen gas and fluorine is reduced to hydrogen fluoride HF.
Sodium Fluoride is an inorganic salt of fluoride used topically or in municipal water fluoridation systems to prevent dental caries. Fluorine is a chemical element with the symbol F and atomic number 9. Fluorine exists as a diatomic gas and its chemical formula is.
As the most electronegative element it is extremely reactive as it reacts with all other elements except for argon neon and helium. In aqueous solution fluorine commonly occurs as the fluoride ion F-. Fluorine F2 CID 24524 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
The chemical symbol for Praseodymium is Pr. 2 KF s F₂ g 2 K sExplanation. The chemical reaction will exist as follows.
In its pure form it is a poisonous pale yellow-green gas with chemical formula F2. Fluorine gas is a univalent poisonous gaseous halogen and in its elemental form we denote it by F_2. H 2 O 2.
Less amount of fluorine gas is fed slowly to water this will give hydrogen fluoride and oxygen gas. Atomic fluorine is univalent and is the most chemically reactive and electronegative of all the elements. Atomic fluorine is univalent and is the most chemically reactive and electronegative of all the elements.
Fluorine and thallous chloride react violently melting the product Mellor Supp. This agent may also inhibit acid production by commensal oral bacteria. Balance Fluorine AtomsAs there is one fluorine atom on left hand side and two fluorine atoms on right.
Whereas calcium exists in solid state and has a chemical formula Ca. What is the Formula for Hydrogen Peroxide. Hydrofluorocarbons HFCs perfluorocarbons PFCs sulfur hexafluoride SF 6 and nitrogen trifluoride NF 3.
Selenium silicon or sulfur ignites in fluorine gas at ordinary temperatures Mellor 211-131946-1947. Fluorine is a diatomic gas. It is so reactive that glass metals and even water as well as other substances burn with a bright flame in a jet of fluorine gas.
In nature fluorine occurs mainly in the minerals fluorspar left CaF_2 right and cryolite left Na3AlF_6 right. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic structure. Therefore this reaction is a redox reaction.
Place your substances accordingly to get. Fluorinated gases F-gases are man-made gases that can stay in the atmosphere for centuries and contribute to a global greenhouse effect. Fluorine readily forms compounds with most other elements even with the noble gases krypton xenon and radon.
The element Fluorine is a poisonous gas. Its atomic mass is 18998403 u. Therefore when fluorine gas reacts with calcium metal then it results in the formation of compound calcium fluoride.
In its pure form it is a poisonous pale yellow-green gas with chemical formula F2. Chemical Formula of Potassium Fluoride KF Chemical Formula of Fluorine Gas F₂ Chemical Formula of Solid Potassium KStep 1. Fluorine is the chemical element in the periodic table that has the symbol F and atomic number 9.
The formula for fluorine gas is F 2. All of the elements that are gases at room temperature besides the noble gases are diatomic elements. While Fluorine gas is an elemental form of the element fluorine at standard.
The fluorine-containing mineral fluorspar fluorite CaF 2 has been used for centuries as a flux cleansing agent in various metallurgical processes. There are four types. It is the lightest halogen and exists at standard conditions as a highly toxic pale yellow diatomic gas.
For your reaction you have. Atomic fluorine is univalent and is the most chemically reactive and electronegative of all the elements. In its pure form it is a poisonous pale yellow-green gas with chemical formula F₂.
Write the unbalance chemical equation KF F₂ KStep 2. The chemical formula of flurine is F₂. F2g Cas CaF2s.
