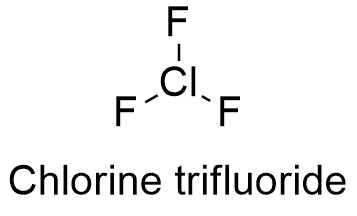
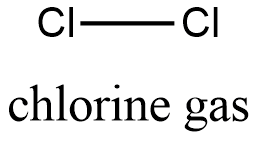After turning red both papers are then bleached white. In this video well write the formula for Chlorine gas.
In addition we sell it as a liquefied compressed gas.

Formula for chlorine gas. The combination of bleach sodium hypochlorite with acid produces chlorine gas a heavy green-yellow gas with a strong odor. It is yellow-green in colour and has an odour which is similar to to the household bleach. For Chlorine gas we need to know that there are two Cl atoms bonded in gases like Oxygen gas O 2 Fluorine gas F 2 Bromine gas Nitrogen gas.
Chlorine gas has also been used as an industrial solvent and has other industrial uses such as the production of bulk materials bleached paper products plastics such as PVC and solvents. Preparation of chlorine gas. It is an extremely reactive element and a strong oxidising agent.
It is greenish-yellow with a pungent suffocating odor gas. Cl2 chlorine never exist in nature but if chlorine is produce in chemical reactions it will bond to each other like oxygen and nitrogen to form a diatomic molecule. Physical properties of chlorine gas.
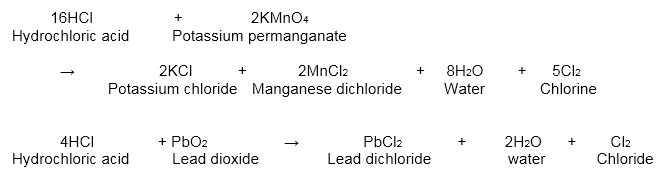
HCl MnO 2 MnCl 2 Cl 2 2H 2 O. Preparation of chlorine gas. We can prepare chlorine by combining hydrochloric acid and manganese dioxide.
7 The chemical formula for chlorine is Cl 2 and its molecular weight is 7090 gmol. The chemical structure of chlorine gas is Cl2 and its molecular weight is 70 gmol. Chlorine gas Cl2 - Chlorine is the chemical element with the formula Cl2 commonly used as disinfectant.
2 Chlorine gas has a pungent choking smell. Among the elements it has the highest electron. The reaction just shows the elements in their standard states their phases at room temperature at 1atm pressure for example chlorine is a gas at room temperature Cl2 bromine is a liquid.
Chlorine gas is an inorganic gas that is utilised in controlled quantities in the chemical industry. Chlorine is an element. Study it and answer the questions that follow.
In addition we sell it as a liquefied compressed gas. Its molar mass is 70906 grams per mole. An explanation of how to balance and write the equation for Sodium Chlorine gas and the correct coefficients for each substance in the equationTo balance.
HCl MnO 2 MnCl 2 Cl 2 2H 2 O. Reactive oxygen species ROS such as superoxide O 2 hydrogen peroxide H 2 O 2 and potentially hydroxyl radical can be formed both via recruited. Ii The equation for the redox reaction that takes place is MnO 2s 4 HCl aq MnCl 2 aq 2H 2 O l Cl 2 g Explain using oxidation.
Chemical compound of the family of halogens chlorine is a yellowish-green gas with suffocating smell very unpleasant and it is extremely toxic Chlorine 17Cl. Chlorine gas is an inorganic gas that is utilised in controlled quantities in the chemical industry. Also it converts to liquid at 35 o C and is slightly soluble in water.
3 Chlorine gas turns moist litmus paper from blue to red and moist universal indicator paper to red - it is acidic. The odor threshold is 031 ppm. What is the chemical formula for chlorine gas.
I Complete the set up to show how dry chloride gas may be collected. Also it converts to liquid at 35 o C and is slightly soluble in water. It is greenish-yellow with a pungent suffocating odor gas.
4 Chlorine gas will put out a lit splint. Hint for Writing the Formula for Chlorine gas. The chlorine gas formula is Cl 2.
What are the General Tests for Chlorine Gas. Chlorine gas phase is the result of combining two chlorine Cl atoms or the formula Cl 2. Chlorine is a yellow-green gas at room temperature.
Visit BYJUS to understand the properties structure and its uses. Its diatomic molecular form is indicated by the symbol Cl2. For Chlorine gas use the hints and resources below to help write the formula.
The chemical formula of chlorine gas is Cl 2. These reactions show the direct combination of the elements chlorine gas and sodium to form NaCl. Hydration of chlorine gas Cl 2 leads to formation of HCl and HOCl hypochlorous acidAs indicated both Cl 2 and HOCl can react with airway lining constituents.
Chlorine Cl2 is NOT the same as the chloride ion Cl-. It is soluble in water and reacts to form hypochlorous acid and hydrochloric acid. Mgm 3 ppm molecular weight of the.
Chlorine is a chemical element with the symbol Cl and atomic number 17. We can prepare chlorine by combining hydrochloric acid and manganese dioxide. To convert concentrations in air at 25C from ppm to mgm 3.
It exists as a yellow-green gas at standard temperature and pressure and its density is 32 grams per liter. Structure properties spectra suppliers and links for. 1 Chlorine gas Cl 2 g is green-yellow in colour.
Physical properties of chlorine gas. Postulated mechanisms for airways injury due to chlorine inhalation. Chlorine gas is an inorganic gas polemic for being used as chemical weapons it is mostly used in controlled quantities in chemical industry.
4 Chlorine has a suffocating odor. Chlorine is a greenish-yellow gas that is slightly soluble in water. Note that Chlorine gas is one of the seven major diatomic gasesThe seven major diatomic elements in.
The chemical formula for chlorine gas is Cl 2. The second-lightest of the halogens it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them.

Chlorine Gas Formula Chemical Formula Of Chlorine Gas On Byju S
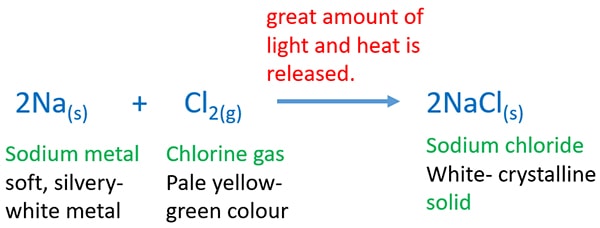
Sodium And Chlorine Gas Reaction Na Cl2

How To Balance Cl2 H2o Hcl O2 Chlorine Gas Water Youtube

Chlorine Gas Test How Does Chlorine React With Water

Write The Formula For Chlorine Gas Youtube

How To Balance Si Cl2 Sicl4 Silicon Chlorine Gas Youtube
Chlorine Atom Images Stock Photos Vectors Shutterstock
Chlorine Preparation Properties Chlorine Poisoning Uses And Videos